News release
From:
Expert Reaction
These comments have been collated by the Science Media Centre to provide a variety of expert perspectives on this issue. Feel free to use these quotes in your stories. Views expressed are the personal opinions of the experts named. They do not represent the views of the SMC or any other organisation unless specifically stated.
Professsor Gareth Baynam is a Clinical Geneticist and Rare Disease Researcher at Telethon Kids Institute.
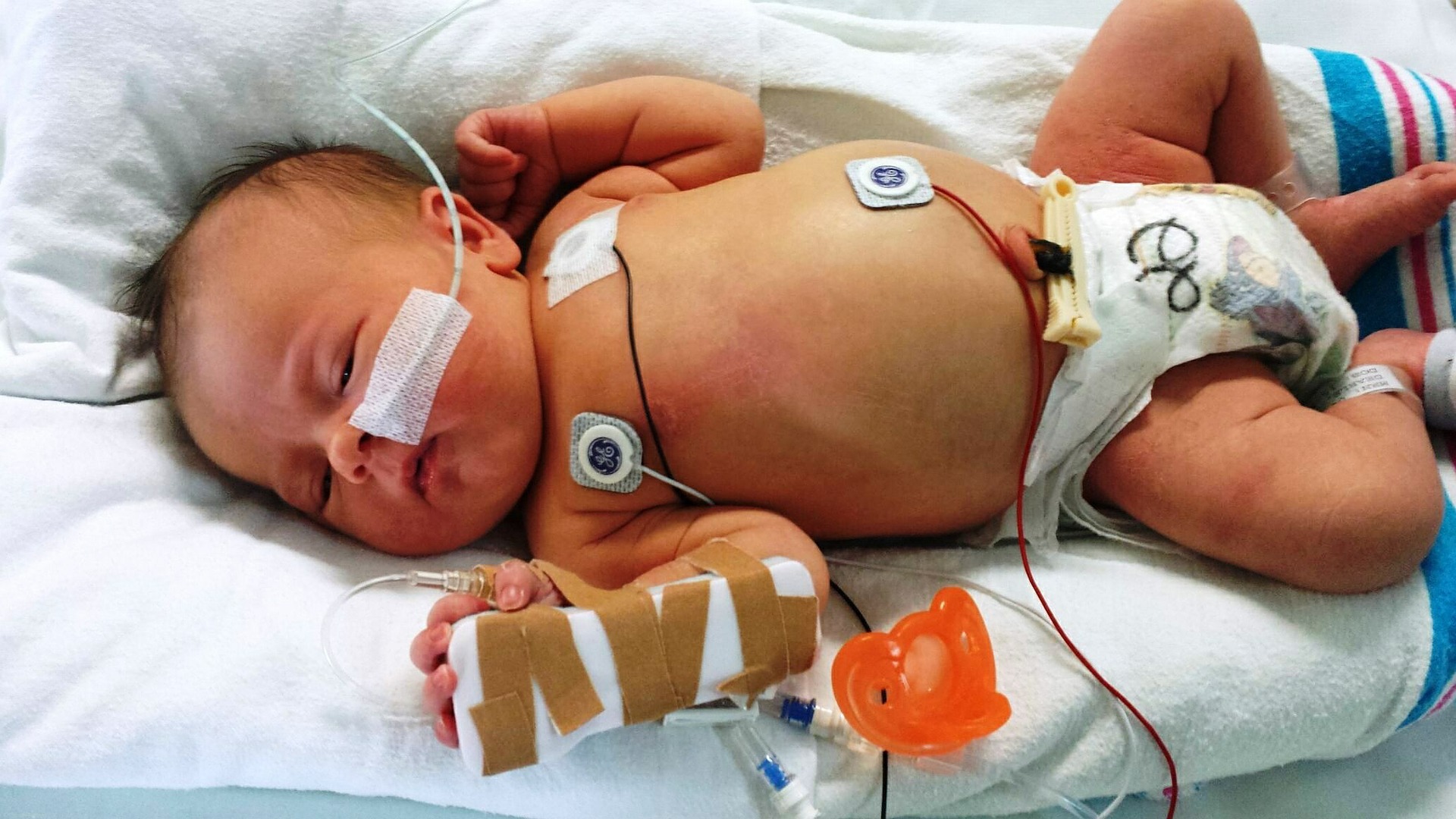
This study of testing in a public health system, together with others, reinforces that undeniably many of our most vulnerable and sickest children have an underlying rare genetic disease as the cause of their condition, from very sick babies in the intensive care unit through to older children with the most complex chronic conditions affecting multiple parts of their bodies. Also that frequently, identifying the underlying rare genetic cause changes medical practice and provides clarity for families. It is critical to continue to advance our states public health system clinical genetic testing and counselling capacity, and its partnership to research. Continuing to improve access to testing for our acutely and chronically sickest children - those that need it most - is a public health need.
Dr Nina McCarthy is a Research Fellow in the Genetic Origins of Health and Disease at the University of Western Australia
This study builds on previous, single-centre studies by examining the feasibility of genomic testing in a national (multi-centre), highly coordinated genomic diagnosis program in a public health care system.
In the critical care setting, genomic testing results need to be delivered rapidly to be useful. The main aim of the study was to evaluate how quickly the genomic tests were performed. The authors found that the average time taken to conduct the test by the genomic laboratories was 3 days, well within their target of 5 days. However, the average total time from hospital admission to genomic report was 17.5 days, with the longest component being time from hospital admission to to test initiation. This suggests that further efforts to speed up the process need to focus on changing clinician practice rather than shortening laboratory turnaround times.
The study also found that genomic testing led to a diagnosis in 51 per cent of the 108 patients included in the study. The genomic testing led to changes in clinical management for 44 per cent of the patients, and was perceived by clinicians as contributing to the clinical management of the patients regardless of whether a molecular diagnosis was made."
My opinion of this research:
"Recent technological improvements have led to improved accuracy and reduced cost of ‘Genomic testing’ (usually involving sequencing of all, or a part of a persons DNA), making it an accessible component of clinical care.
I think that studies such as these are vitally important to ensure that we implement genomics in healthcare in a responsible and equitable way. Although I believe that genomic medicine holds a huge amount of promise, it also has the potential to be very damaging if its not implemented well. Often the results of genomic tests are not clearcut, and require careful interpretation and communication by appropriately trained health professionals in what are often emotionally charged and rapidly evolving clinical situations.
I think that the approach taken by this study, of leveraging the Australian Genomics Health Alliance (a government-funded national genomic medicine initiative) to build national governance and infrastructure for the implementation of genomic testing, is a sensible one.
As the authors note, it is an extremely challenging undertaking which requires multidisciplinary teams including physicians, clinical geneticists, genetic counsellors, laboratory scientists, and bioinformaticians simultaneously changing practices across multiple sites to provide consistent service. However, I think it’s important that we not jump the gun with genomic testing, and put in the work necessary to ensure that appropriate frameworks are in place.
Professor Jozef Gecz is an NHMRC Senior Principal Research Fellow and the Channel 7 Children’s Research Foundation Chair for the Prevention of Childhood Disability, at the University of Adelaide
It has been two decades since the international scientific community decoded the first complete Book of Life, the DNA sequence of the first human genome. It cost USD$3 billion. By 2020 millions of individual human genomes have been ‘read’ and the information gained is revolutionising precision health for everyone. Critically ill children and their families are among the next to benefit when DNA sequencing comes to their bedside. The team of the Australian Genomics Health Alliance led by Dr Zornitza Stark from the Melbourne Children’s Research Institute reports on their success (JAMA) with prospective, ultra-rapid DNA sequencing of 108 children from primarily neonatal and paediatric intensive care units from 12 Australian hospitals. These critically ill infants were on average less than one month old! While the 51 per cent molecular diagnostic success rate is a great achievement on its own, it is the speed of just 3.3 days from sample receipt to DNA diagnostic report, which is simply amazing. For many infants (42/55) the diagnosis led to a change in their clinical management. A test like this would otherwise take weeks or months to complete. While the ultra-fast DNA testing is not yet a standard of care in Australia, this study paves a major way towards it.



 Australia; NSW; VIC; QLD; SA
Australia; NSW; VIC; QLD; SA