News release
From:
Developmental biology: Human blastocyst-like structures generated in a dish (N&V) *PRESS BRIEFING*
The generation of human blastocyst-like structures in the laboratory is described in a pair of papers published in Nature. The findings provide a model for studying early human development, and may lead to insights into early developmental defects, as well as aiding in the development of new in vitro fertilization (IVF)-associated therapies.
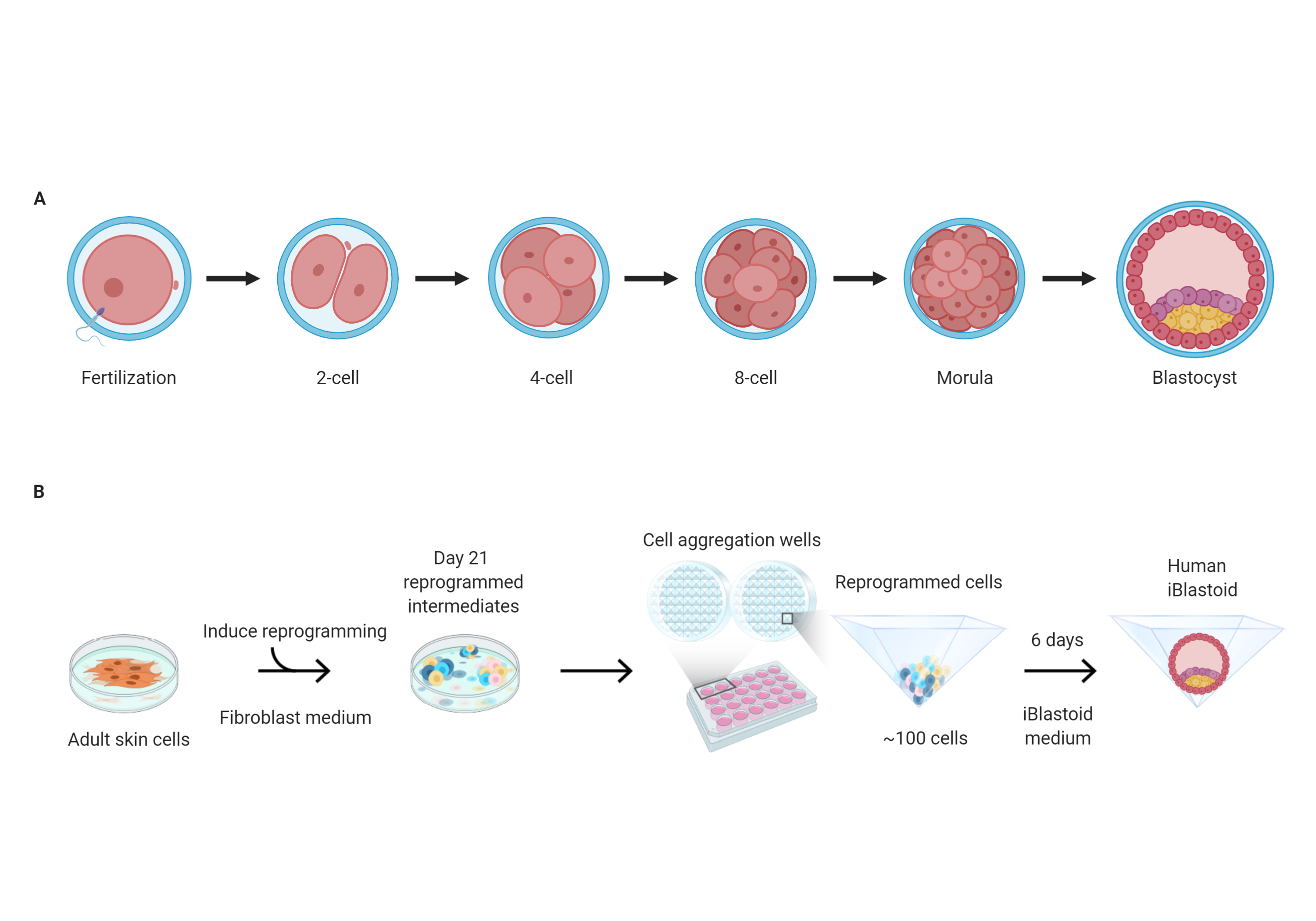
A few days after an egg has been fertilized, it develops into a blastocyst. This is a spherical structure made up of an outer layer of cells surrounding a fluid-filled cavity that contains a mass of embryonic cells. However, our understanding of early human embryonic development has been limited by a lack of appropriate models. Human blastocysts donated to research following IVF have provided insights, but their availability and use is limited. Recently, mouse blastocyst-like structures called blastoids have been produced in the laboratory, which model several aspects of early development in mice. However, the generation of similar blastoids from human cells has not previously been reported.
Jose Polo and colleagues reprogrammed human fibroblasts — the main cell type found in connective tissue — to produce three-dimensional models of the human blastocyst in the laboratory, which they called ‘iBlastoids’ (induced blastoids). The authors found that iBlastoids model the overall architecture of blastocysts and are capable of giving rise to pluripotent and trophoblast stem cells. They were also able to model several aspects of the early stage of implantation. However, they note that the iBlastoids should not be considered as an equivalent to human blastocysts.
In a separate study, Jun Wu and colleagues developed a three-dimensional culture strategy that allowed them to generate blastocyst-like structures, which they term ‘human blastoids’, from human pluripotent stem cells. The human blastoids resembled human blastocysts in their morphology, size, cell number and composition of different cell lineages. Human blastoids are able to generate embryonic and extraembryonic stem cells and can self-organize into structures with features of peri-implantation human embryos. In addition, the authors discovered the role of protein kinase C signaling in blastoid cavity formation. They emphasize that human blastoids are not equivalent to human blastocysts and are unable to give rise to a viable embryo.
Although these two models reproduce key aspects of early human development, they present a number of differences to actual human embryos and therefore should not be considered as such. Yi Zheng and Jianping Fu note in an accompanying News & Views that as protocols are optimized, these blastoids will more closely mimic human blastocysts, raising bioethical questions. “Thus, the continuous development of human embryo models, including human blastoids, calls for public conversations on the scientific significance of such research, as well as on the societal and ethical issues it raises.”
Expert Reaction
These comments have been collated by the Science Media Centre to provide a variety of expert perspectives on this issue. Feel free to use these quotes in your stories. Views expressed are the personal opinions of the experts named. They do not represent the views of the SMC or any other organisation unless specifically stated.
Professor Martin Pera holds the Chair of Stem Cell Sciences at: The University of Melbourne, Florey Neuroscience and Mental Health Institute and the Walter and Eliza Hall Institute of Medical Research. He is also the Program Leader of Stem Cells Australia
Two studies published in Nature today show for the first time that human pluripotent stem cells can give rise to three-dimensional structures that closely mimic the properties of the embryo just around the time it implants into the womb. Jose Polo’s team from Monash University and their collaborators started with stem cells made by reprogramming of adult skin cells, while Jun Wu’s group at the University of Texas Southwestern Medical Center began with human embryonic stem cells.
Both groups created conditions in a petri dish that directed the starting stem cell populations to form the three cell types found in the embryo before implantation: the precursors of the embryo itself, and two types of essential supporting cells that provide for transfer of nutrients to the embryo.
The structures resembled human embryos in their overall appearance and in their molecular makeup. Both groups showed that these embryo models, called iBlastoids after the embryonic blastocyst stage they resemble most closely, could continue to develop in vitro in a fashion that mimicked the further growth and differentiation of the embryo after implantation into the womb.
These model systems have great potential for providing insight into early human development, and for enabling the discovery of new medical approaches to establishing and maintaining a healthy pregnancy.
While the blastoids described by both groups are excellent models, they do differ in some respects from normal embryos.
The studies were performed under strict ethical oversight, but they will undoubtedly prompt further discussion regarding what work should be permitted with embryo models in a dish, particularly as they become more refined and capture later stages of human development.
To help inform these discussions, the International Society for Stem Cell Research has considered these questions carefully, and will soon be issuing guidelines for the conduct of embryo model research.
Dr Jason Limnios is Leader of the Pluripotent Stem Cell and Retinal Cell Development lab at the Clem Jones Centre for Regenerative Medicine, Bond University.
Natural human development, IVF and cloning (think 'Dolly the sheep') all have one thing in common: the need for an egg to begin development. These studies show two remarkable things: that the earliest stages of human development do not need an egg, and that it can be achieved using only skin cells, a few genes, and the right chemical conditions.
iBlastoids now allow stem cell biologists and embryologists to better understand human development without using human embryos. iBlastoid research will likely provide insight for IVF culture which can be used to improve the quality of real embryos for assisted reproduction. It will also allow scientists to study the effect of genetics, chemical exposure, viruses, and other factors on human development, and may help lead to the discovery of new drug and cell therapies.
The public will have probably a range of feelings, opinions and questions about new embryonic and stem cell technologies. I understand that. I grew up in a Christian home and had these conversations with my family. However, it is really important for all to understand that iBlastoids aren't normal human embryos and won't make a baby if implanted. There are also very strict laws about growing anything like a human embryo in the lab. Both groups took great care to make sure that they stayed within the 14-day limit and stopped experiments before the first sign of embryonic change (gastrulation).
Professor Megan Munsie is from the School of Biomedical Sciences and Melbourne Medical School at the University of Melbourne. She heads a research program in the ethical, legal and social implications of stem cell research.
If you have undergone infertility, or know someone who has, you may recognise the term blastocyst. You may even be able to describe what it looks like. This microscopic fluid-filled spherical structure of around 200 cells has a simple but very characteristic structure. It is the stage of development just before implantation and when IVF embryos are transferred to the uterus. If a pregnancy is established, and for many that remains a big if, cells on the outside will give rise to the placenta and those clustered as a mass inside the sphere will develop into the fetus.
For the first time researchers have been able to use stem cells and related reprogramming technology to create 3D structures that mimic the developing human blastocyst – a stage that is very poorly understood. These structures referred to as blastoids promise a new way to study how the cells at this very early stage of development interact, potentially leading to improvements in infertility treatments and ways to prevent pregnancy loss.
While these models are similar, they should not be considered equivalent to human embryos created in an IVF lab. This distinction is important. Ethical and legal considerations are different. How and when they are used in research is likely to be different. However, for many in the community – hearing about these new discoveries for the first time – this may not be obvious.
In a welcomed move, the International Society for Stem Cell Research will soon release updated guidelines that will specifically address the use of human embryo models and other advances in stem cell research.
Like many other areas of stem cell research, it is vital that we continue to discuss the science, its value and how ethical issues will be address as the field evolves.
A/Professor Kuldip Sidhu is co-founder and director, CK Cell Technologies and Conjoint with University of New South Wales Medicine.
Since 1978 with the birth of the first test-tube baby, more than 8 million babies have been born with assisted reproduction to-date. In Australia, about one in twenty is born using this technique. The success rate varies but is consistently high, above 35 per cent worldwide, which is more than with natural cycles in women.
Assisted reproduction also brings in concern about inherent birth defects and ailments in the babies associated with this procedure. So it’s very timely that these two papers in Nature explicitly describe a procedure to generate iblastoids from pluripotent stem cells that resemble natural blastocysts and claim that the same can be utilised to study early human development and the ailments associated thereof.
Although the efficiency of the procedure remains low, nevertheless it represents a novel in vitro model for early human development. Authors of both these papers could generate two types of stem cells, epiblast and trophectoderm cells, by using ‘whole iblastoids culture’ but it will be pertinent to demonstrate that epiblast from these iblastoids can be dissected out and the relevant naïve epiblast stem cells are generated to validate close homology with blastocysts.
These studies set a solid ground to optimise this system further as a tool to assess the early human development and more including the ailments that are associated with assisted reproduction.
Multimedia



 Australia; NSW; VIC; QLD; WA
Australia; NSW; VIC; QLD; WA