News release
From:
Expanding access to testing and treatment for hepatitis B
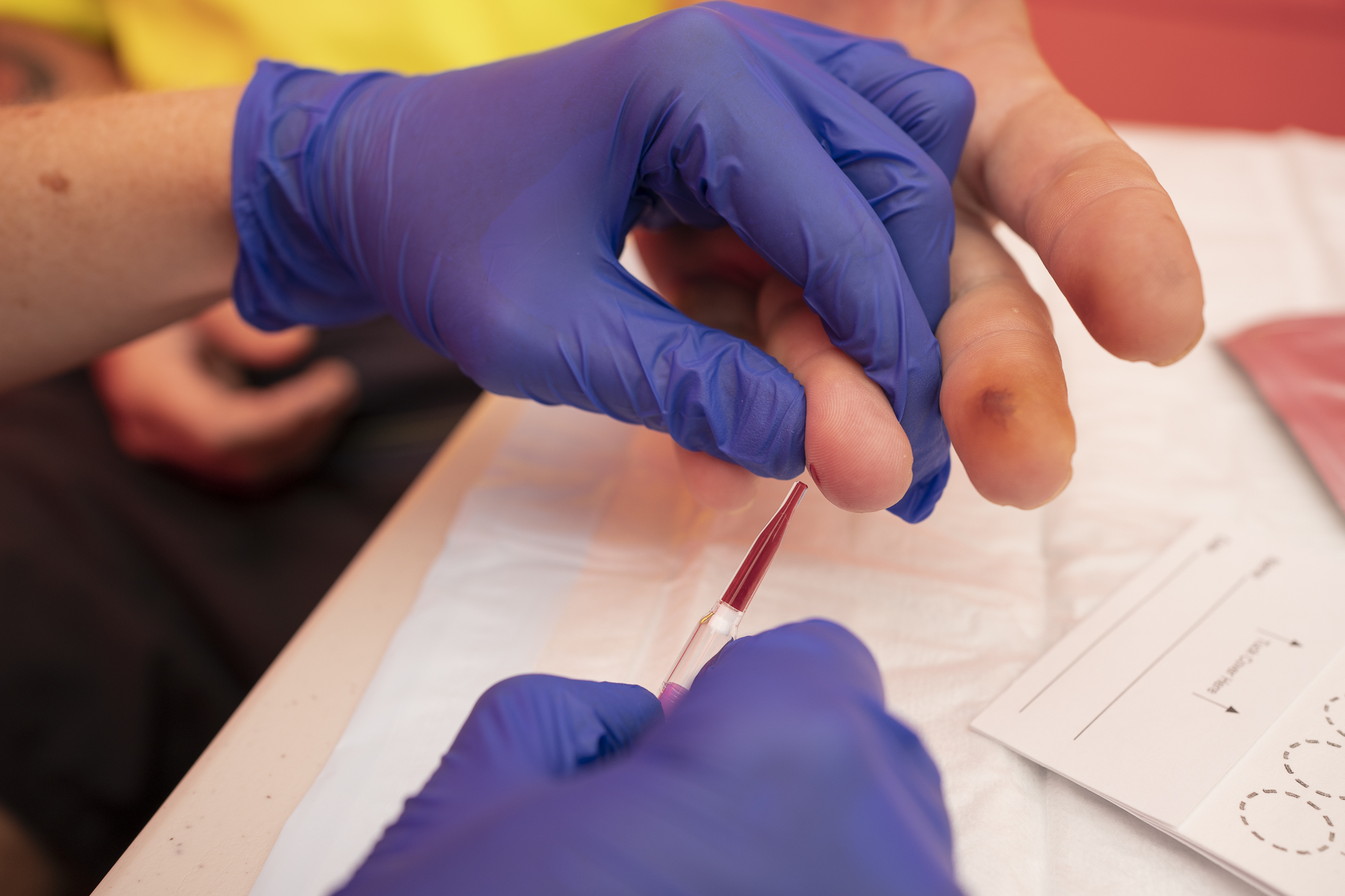
A clinical trial led by Kirby Institute at UNSW Sydney has found that point-of-care testing for hepatitis B DNA is as effective as traditional laboratory testing, paving the way for faster diagnosis and treatment in hard-to-reach communities. The results have been published in the Journal of Clinical Microbiology.
“The results of our trial found that the fingerstick point-of-care test is highly accurate, closely matching the accuracy of traditional tests,” explains Professor Gail Matthews who led the research at the Kirby Institute. “This is a very important finding because it has the potential to expand access to testing and treatment globally, and especially in resource limited settings or remote areas, where current testing access is poor.”
Hepatitis B is a viral infection that attacks the liver, causing inflammation and, over time, serious complications such as cirrhosis, liver failure, and liver cancer. It is responsible for over 1 million deaths per year, but is preventable by vaccination, and effective treatment is available for chronic infection.
While most high-income countries have strong vaccination programs and reasonable access to care, the majority of people with chronic hepatitis B live in low- and middle-income countries, where access to testing and treatment is limited. Even in Australia, hepatitis B DNA testing is more difficult to access for people living in remote areas.
"Not everyone who has hep B needs treatment,” explains Associate Professor Behzad Hajarizadeh, who is first author on the paper. “People with higher levels of the virus are more likely to benefit from treatment, so DNA tests are required to determine the levels of virus in the system. DNA testing is also used once a patient starts treatment, to help understand if the treatment is working.”
Currently, hepatitis B DNA testing, for both diagnosis and monitoring, requires collecting a venous blood sample to be processed in centralised laboratories, meaning patients can need to travel long distances to take the test, and then often wait days or weeks for results. This delay, and the multiple clinic visits involved, can hinder timely treatment and care.
By contrast, point-of-care is a type of test that can be done in small health clinics using a finger stick blood sample, which can be performed by a broader range of health care workers, and provides a result within 60 minutes. It is an effective alternative to laboratory testing for many infectious diseases, including hepatitis C, but until now, its efficacy for hepatitis B DNA using finger stick blood has been unknown.
“Our research demonstrates that point-of-care testing for hepatitis B DNA using finger stick blood is, indeed, highly accurate and effective. Given the technology is already in use for a range of other infectious diseases globally, our evidence paves the way for integrating infectious disease care significantly enhancing access to hepatitis B testing, monitoring and treatment, no matter where someone lives,” says the Kirby Institute’s Associate Professor Tanya Applegate.
Most recent World Health Organization figures (2022) estimate that there are 254 million people living with chronic hepatitis B infection worldwide, yet only 14% were diagnosed and just 8% were receiving treatment, representing a major global health challenge. It is currently estimated that no country is on track to meet the WHO target of elimination of hepatitis B as a public health threat by 2030. As part of a push to increase testing and treatment, most recent WHO guidelines include a new recommendation supporting the use of hepatitis B point-of-care DNA fingerstick tests globally. Data from this study supports that recommendation.
“Access to testing is a major barrier to progress on hepatitis B elimination,” says Associate Professor Thomas Tu from Hepatitis B Voices. “We are hopeful that this research will support the roll-out of point-of-care testing for hepatitis B, enhancing access and ultimately, improving health and saving lives.”



 Australia; NSW; VIC
Australia; NSW; VIC