News release
From:
A Griffith University-led research team has discovered how a therapeutic target common among debilitating neurodegenerative disorders is activated, which could help accelerate drug development.
In a study published in the journal Neuron, the researchers from Griffith University’s Institute for Glycomics, the University of Queensland and Washington University, analysed the structure and function of a protein called SARM1, which is involved in the destruction of nerve fibres. They found that the protein is a sensor that responds to the levels of specific molecules derived from metabolism.
“SARM1 is a potential therapeutic target for many neurodegenerative diseases,’’ said lead author and Institute for Glycomics researcher, Dr Thomas Ve.
“When nerve fibres are damaged, whether by injury, disease or as a side effect of certain drugs, SARM1 is called into action which sets off a series of events in the cell that trigger them to self-destruct.
“This destruction likely plays an important role in multiple neurodegenerative conditions, including peripheral neuropathy, Parkinson's disease, amyotrophic lateral sclerosis (ALS), traumatic brain injury and glaucoma.”
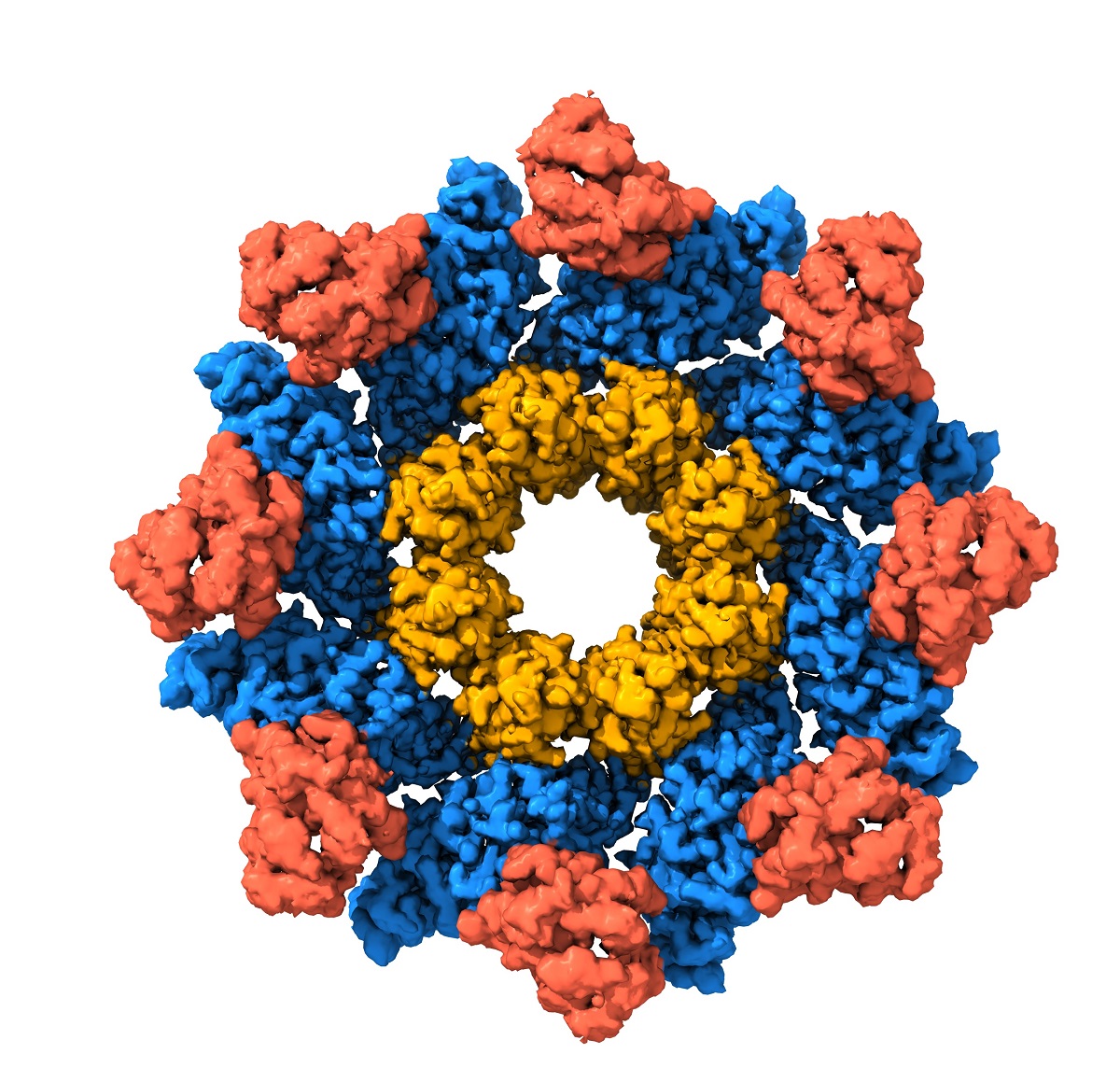
Dr Ve and joint first author on the paper, Dr Yun Shi used NMR spectroscopy, a biophysical tool to analyse interactions between proteins and small molecules, to demonstrate that two important metabolites in nerve cells compete for binding to the SARM1 protein, and the ratio of these two metabolites determines whether SARM1 becomes activated.
Dr Ve and collaborators also used structural biology tools - Cryo-electron Microscopy and X-ray Crystallography - to determine three-dimensional structures of the SARM1 protein which enabled them to pinpoint exactly where these two metabolites bind to SARM1 and how they regulate SARM1 activation.
Dr Ve, also an Australian Research Council Future Fellow and NHMRC Investigator, said the new structural information about SARM1 had the potential to accelerate the development of drugs that target neurodegenerative diseases.
“We are very excited by these findings as they greatly advance our understanding of how SARM1 is activated,’’ he said.
“It provides clues as to how one might block activation of this protein using structure-guided approaches to prevent nerve fibre loss in neurodegenerative diseases.”
Professor Mark von Itzstein AO, Director of the Institute for Glycomics, welcomed this important breakthrough. “New strategies towards solving neurodegenerative diseases have become increasingly important due to the enormous impact on the quality of life of those that suffer with these conditions”.
The Griffith team worked in collaboration with the group of Professor Bostjan Kobe at University of Queensland, and the groups of Professors Aaron DiAntonio and Jeffrey Milbrandt at Washington University, St Louis, USA. It also involved Dr Ve’s industry partner Disarm Therapeutics, a wholly-owned subsidiary of Eli Lilly & Co, whose mission is to create breakthrough disease-modifying therapeutics to treat patients affected by axonal degeneration.



 Australia; QLD
Australia; QLD