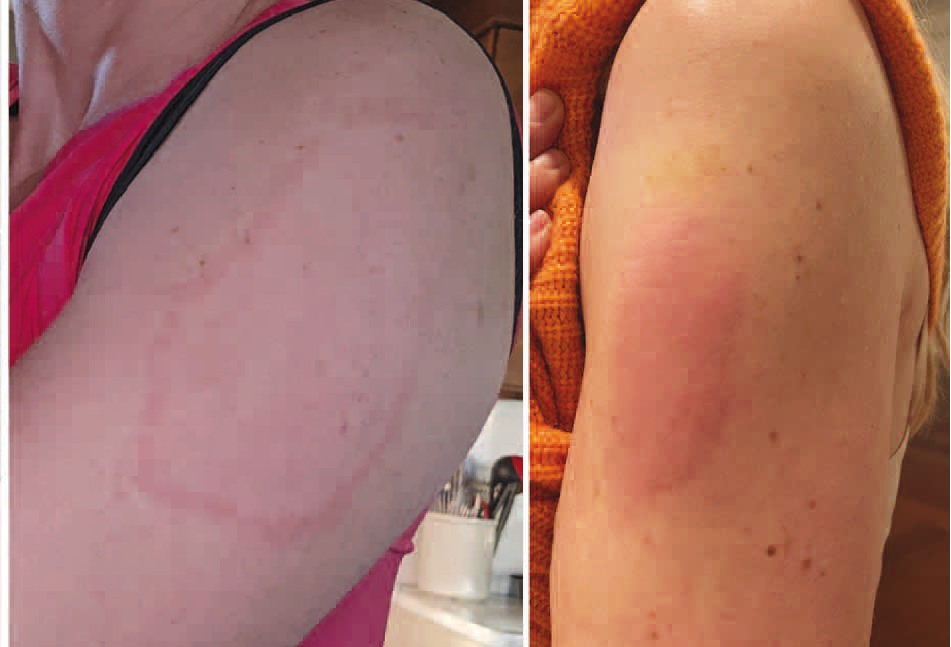
Attachments
Note: Not all attachments are visible to the general public. Research URLs will go live after the embargo ends.

Journal/
conference: JAMA Dermatology
conference: JAMA Dermatology
Research:Paper
Organisation/s:
Yale University School of Medicine, USA
Funder:
Conflict of Interest Disclosures: Dr Little reported a grant from the National Center for Advancing Translational Science (CTSA No. KL2 TR001862), which is a component of the National Institutes of Health, and aWomen’s Health Career Development Award from the Dermatology Foundation during the conduct of the study. DrWatsky reported equity in Johnson & Johnson held by his spouse’s retirement fund outside the submitted work. No other disclosures were reported.



 International
International