News release
From:
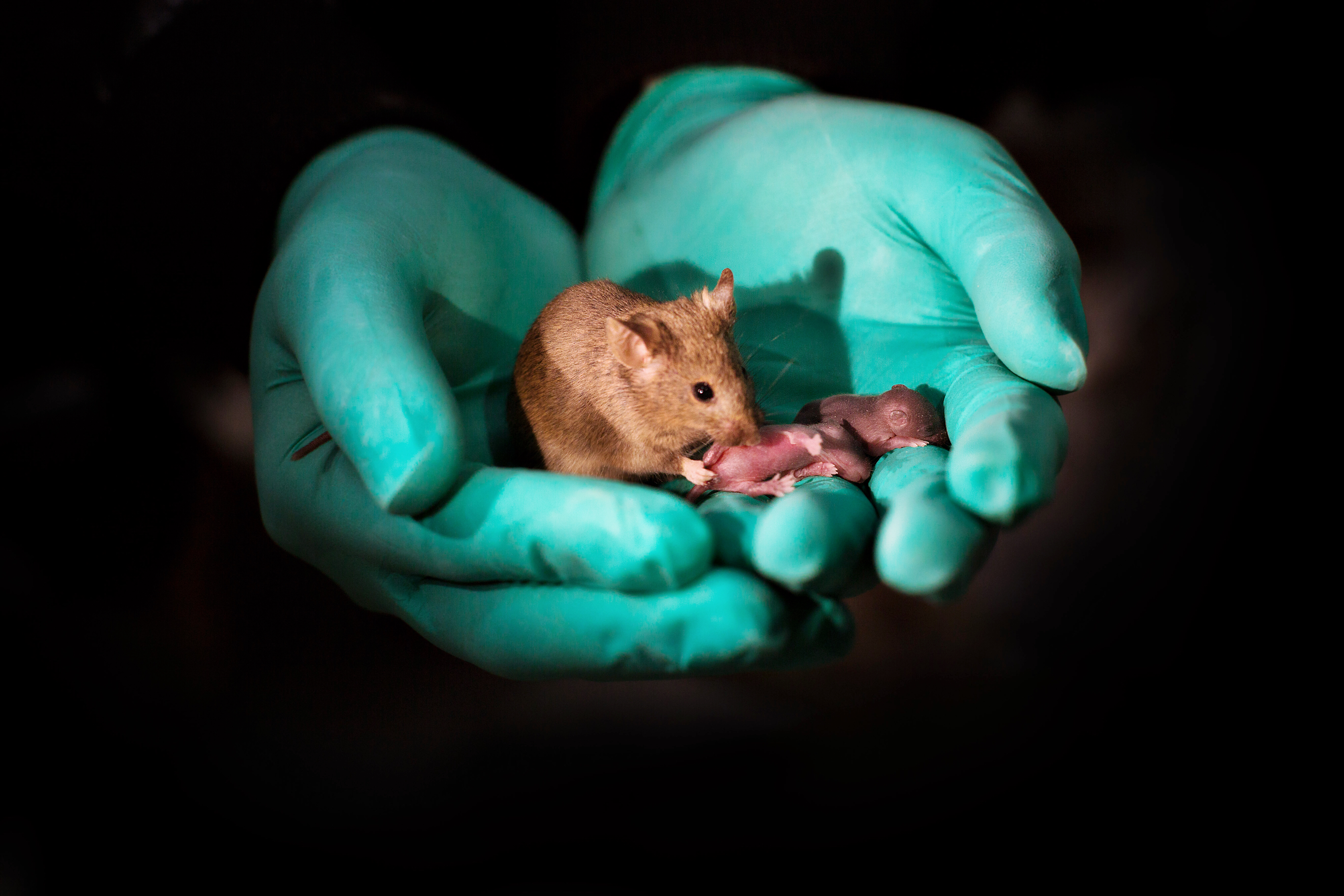
Mouse pups with same-sex parents born in China using stem cells and gene editing
Researchers at the Chinese Academy of Sciences were able to produce healthy mice with two mothers that went on to have normal offspring of their own. Mice from two dads were also born but only survived for a couple of days. The work, presented October 11 in the journal Cell Stem Cell, looks at what makes it so challenging for animals of the same sex to produce offspring and suggests that some of these barriers can be overcome using stem cells and targeted gene editing.
"We were interested in the question of why mammals can only undergo sexual reproduction. We have made several findings in the past by combining reproduction and regeneration, so we tried to find out whether more normal mice with two female parents, or even mice with two male parents, could be produced using haploid embryonic stem cells with gene deletions," says co-senior author Qi Zhou.
While some reptiles, amphibians, and fish can reproduce with one parent of the same sex, it's challenging for mammals to do the same even with the help of fertilization technology. In mammals, because certain maternal or paternal genes are shut off during germline development by a mechanism called genomic imprinting, offspring that don't receive genetic material from both a mother and a father might experience developmental abnormalities or might not be viable. By deleting these imprinted genes from immature eggs, researchers have produced bimaternal mice--mice with two mothers--in the past. "However, the generated mice still showed defective features, and the method itself is very impractical and hard to use," says Zhou.
To produce their healthy bimaternal mice, Zhou, co-senior author Baoyang Hu, co-senior author Wei Li, and their colleagues used haploid embryonic stem cells (ESCs), which contain half the normal number of chromosomes and DNA from only one parent and which the researchers believe were the key to their success. The researchers created the mice with two mothers by deleting three imprinting regions of the genome from haploid ESCs containing a female parent's DNA and injected them into eggs from another female mouse. They produced 29 live mice from 210 embryos. The mice were normal, lived to adulthood, and had babies of their own.
One advantage of using haploid ESCs is that even before the problematic genes are knocked out, they contain less of the imprinting programming that ultimately causes maternal- or paternal-specific genes to be expressed. "We found in this study that haploid ESCs were more similar to primordial germ cells, the precursors of eggs and sperm. The genomic imprinting that's found in gametes was 'erased,'" says Hu.
Twelve live, full-term mice with two genetic fathers were produced using a similar but more complicated procedure. Haploid ESCs containing only a male parent's DNA were modified to delete seven key imprinted regions. The edited haploid ESCs were then injected--along with sperm from another male mouse--into an egg cell that had its nucleus, and therefore its female genetic material, removed. This created an embryo containing only genomic DNA from the two male parents. These embryos were transferred along with placental material to surrogate mothers, who carried them to term.
These pups survived 48 hours after birth, but the researchers are planning to improve the process so that the bipaternal mice live to adulthood. Similar results were achieved in 2011 but using a method that relied on a female intermediary produced from the first father's stem cells to mate with the second father. That method sidestepped the problem of genomic imprinting but presents ethical and practical hurdles if this technology were to ever be considered for humans.
Li notes that there are still obstacles to using these methods in other mammals, including the need to identify problematic imprinted genes that are unique to each species and concerns for the offspring that don't survive or that experience severe abnormalities. They do hope, however, to explore these techniques in other research animals in the future.
"This research shows us what's possible," he says. "We saw that the defects in bimaternal mice can be eliminated and that bipaternal reproduction barriers in mammals can also be crossed through imprinting modification. We also revealed some of the most important imprinted regions that hinder the development of mice with same sex parents, which are also interesting for studying genomic imprinting and animal cloning."
Expert Reaction
These comments have been collated by the Science Media Centre to provide a variety of expert perspectives on this issue. Feel free to use these quotes in your stories. Views expressed are the personal opinions of the experts named. They do not represent the views of the SMC or any other organisation unless specifically stated.
Dr Tim Hore, Senior Lecturer, Department of Anatomy, University of Otago
This is an interesting paper following-up on a long-standing question of developmental biology - why is it that in mammalian newborns you need to have equal genetic contributions from both a mother and a father, whereas elsewhere in the animal kingdom, it is possible to create (for example), chickens, komodo dragons and sharks without a genetic contributions from a father?
"It turns out that mammals express some of their genes only from mother’s DNA, and some only from father’s DNA. Around 100 genes are affected by this peculiar form of parent-gene expression, and because some of the affected genes are essential for proper growth and development, you need to have DNA from both mother and father in order to create healthy mammalian offspring."
What did the researchers do?
"The researchers in this paper used genetic modification to alter the genes which are expressed in a parent-specific manner in mammals. In doing so, they were able to artificially overcome some of the usual incompatibility between parents of the same sex, meaning they were able to create relatively healthy offspring with two-mothers, and somewhat unhealthy offspring from two fathers that died shortly after birth."
What's the significance?
"Mice with two mothers were created in the early 2000s (Kono et al., 2004), however, what makes this achievement unique is the range of technology used – the researchers cleverly combined gene editing and the production of stem cells from only one parent (known as ‘haploid’ stem cells). Yet, the work does fall short of creating mammalian offspring from the same sex in the absence of substantial genetic modification, meaning it is unlikely to be useful in humans – for now.
"In order for same-sex parents to both have genetic contributions to their children in an assisted reproduction setting, it is likely another technological leap will be required. One possible approach is using ‘epigenetic-editing’ on haploid stem cells, essentially reprogramming the DNA of one parent so it looks like that of the opposite sex without altering any genetic sequence.
Dr Teresa Holm, Research Fellow, Department of Molecular Medicine and Pathology, University of Auckland
This is an important advance that builds on previous efforts to make uniparental (‘fatherless’ or motherless’) offspring using DNA from same-sex parents. Improving on prior studies, researchers from the Chinese Academy of Sciences generated uniparental mice by genetically modifying embryonic stem cells and inserting the DNA into unfertilised eggs.
This process of reproduction by same-sex parents is not normally possible in mammals due to a phenomenon known as ‘imprinting’, whereby certain genes are turned on or off depending on whether they come from the mother or father. If these ‘gene dosage’ effects are not tightly controlled it can be catastrophic for the embryo, resulting in birth defects or death.
"In this study, some of the key imprinted DNA regions were removed by gene editing, resulting in healthy female mice but males that died shortly after birth.
"The major impact of this work is the furthering of our fundamental understanding of how imprinting operates in mammals and how it acts as a barrier to uniparental reproduction.
"In the long-term, this knowledge may help researchers improve assisted reproductive technologies for infertile couples where disturbances in imprinting may contribute to the health of artificially fertilised embryos. It may even lead to the development of ways for same sex couples to reproduce healthy of their own children. However, it is important to note that the current work was carried out in mice and involved a number of genetic modifications in embryonic stem cells.
"Therefore, this kind of approach in humans carries significant ethical and safety concerns that would need to be overcome if it was to move beyond the laboratory.
Robert Norman is Director of the Robinson institute and Professor of Reproductive and Periconceptual Medicine at the University of Adelaide
This is a paper that deals with fundamental questions about reproduction looking at the use of two females or two males to make an embryo and live animal.
This can occur naturally in non-mammalian animals but not in more evolutionary advanced species.
These investigators have made egg and sperm like structures from stem cells and used two females or two males to make embryos (in contrast to nature where one male and one female are used).
To succeed they had to modify the chemical groups that modulate the DNA expression known as epigenetic markers.
This approach led to a successful outcome in female-female fertilisation but less so in males.
The female offspring were able to reproduce, had a longer lifespan, different cognitive and behavioural changes and some metabolic abnormalities.
This approach is still not perfect but may lead to successful outcomes for endangered species of animals and possibly better experimental research animals.
The concept is intriguing for human reproduction, particularly for same-sex couples but there are far too many uncertainties at present to attempt such an approach for many years to come.
The first challenge will be to make babies from artificially derived eggs and sperm from male and female couples, an exercise that is increasingly important for infertile men and women who have no functioning gametes of their own. No ethical permission has been given anywhere to attempt to produce live offspring although embryos have been produced experimentally with no transfer to the uterus.
Professor Bob Williamson is Chair of the Board of Stem Cells Australia and Scientific Director of the Yulgilbar Alzheimer's Research Program at the University of Melbourne
Powerful new research techniques can mutate genes easily, even in egg and sperm cells. Other techniques can generate the equivalent of early embryo stem cells from blood or skin. This Chinese group has shown that, in mice, a few quick gene mutations can change the usual restrictions on parents, so that two females can be the parents of a baby mouse.
It is easy to joke about this, or to think of its attractiveness to same sex couples. If the research is reproducible, and also works in humans, it still has to be shown to be safe. In Australia, there are both legal prohibitions and ethics committees that would ensure these techniques could not be used for human reproduction. The experiments are, however, important, because they may shine a light on some causes of serious handicap in children.
This research does illustrate how quickly research with stem cells is moving, and everyone, whether they feel enthusiastic or worried about these new techniques, will be thinking (no doubt along with the Science Minister): “Surely, we should have an ethics group that studies this, and can advise the Government if these changes are moving too far, too fast.”
Associate Professor Megan Munsie is Deputy Director of the Centre for Stem Cell Systems at The University of Melbourne and Head of Engagement, Ethics and Policy at Stem Cells Australia
Reproduction in mammals is complex. In their new paper, Qi Zhou and colleagues from the Chinese Academy of Sciences challenge the notion that creating mammalian offspring requires inheritance of a set of chromosomes from a mother and a father. By employing a complicated combination of gene editing, stem cells and reproductive technologies, they showed that it was possible to create what appears to be normal mice using the genetic material from two mothers. Using a similar but slightly more complex process they also created pups from two fathers but these died within days of birth, while others had significant abnormalities.
While this finding provides insights into the control of reproduction, being able to routinely make mice and other mammals from same-sex parents will remain a significant challenge. At a technical level, significant refinements will need to be made to avoid growth abnormalities and other complications. It is likely that a different combination of genes will need to be manipulated to allow normal development for each species, with a high cost to pay if the ‘wrong’ genes are manipulated. Large numbers of surrogates and donors of eggs and embryos would also be required. For male same parent attempts, creating offspring requires genetic material and gametes from far more than two parents raising substantial challenges around pursuing this approach.
The paper is a timely reminder of the power of technology to explore how development and inheritance is controlled. However, it is also a reminder of the need to discuss the potential impacts of technology on society, even when at such a preliminary stage. We need to pause and evaluate under what circumstances, if any, the technology should be applied and, if so, to whom. Simple headlines about being able to create young from same sex parents fails to fully capture the complexity and challenges ahead.
More on the technology: It had been thought that reproduction in mammals was predicated on receiving DNA from both maternal and paternal origin as eggs and sperm have a different yet complementary pattern of coding that controls gene expression. This patterning is referred to as imprinting and is imposed during sperm and egg development in mammals. The researchers used mouse embryonic stem cells that contained only a single set of chromosomes (haploid) rather than the pairs that are usually present in mouse cells. They could then delete several regions of the genome (three different regions in the female, and seven regions in the male stem cells) to create stem cells where imprinting was erased.
To create pups from solely female genetic material, nuclei from modified haploid female stem cells were injected into normal eggs (from another mouse) to create embryos that, when transferred to a surrogate mouse, could develop, albeit at a low efficiency, into pups (210 embryos created 29 pups).
To create pups from male genetic material, the process was technically more complicated. Again they injected the nucleus from genetically modified male haploid stem cells, alongside sperm from another mouse, into eggs where the female chromosomes were removed. The resulting embryos were then allowed to develop in the lab for several days. However, instead of transferring to surrogates, the researchers first obtained stem cells from these embryos and then combined these ‘second round’ stem cells with other embryos that been altered so that they could only contribute to the placental tissue. Using this extremely complicated route produced two live pups from almost 500 transferred embryos and both died within 48 hours of birth.



 International
International