News release
From:
Researchers from the University of Adelaide have discovered how water molecules are organised during plant hydrolytic reactions, knowledge which could have sweeping consequences for the biomedical, pharmaceutical, food and chemical industries.
The research team, led by Professor Maria Hrmova from the University of Adelaide, identified enzyme components that underlie water molecule networks and function as principal operators to regulate water flux during hydrolytic reactions.
“Conceptually, one of the most exciting undertakings in biophysics and biochemistry is to investigate the dynamics of water molecules,” said Professor Hrmova, whose study was published in Communications Biology.
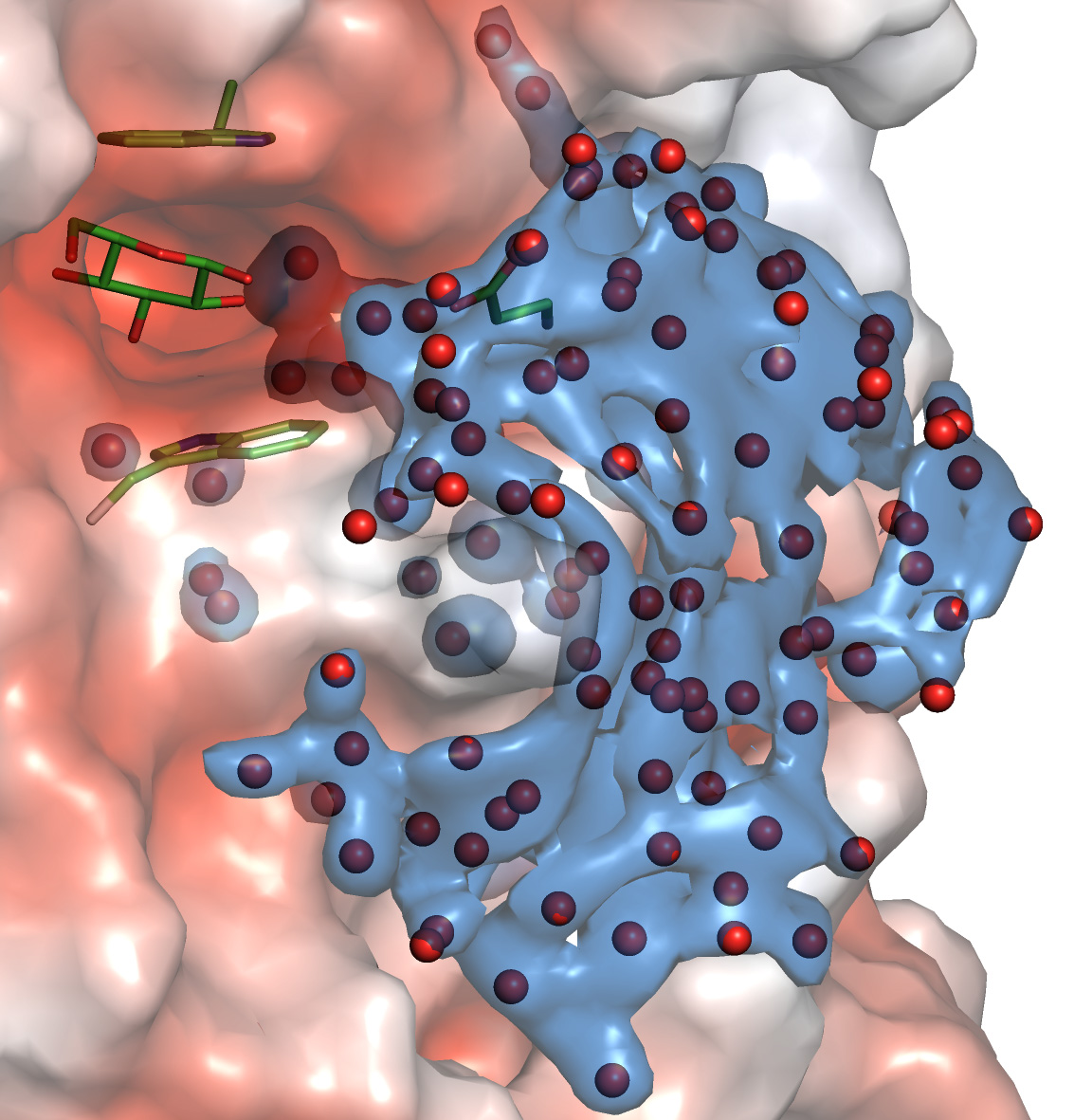
“Water molecules are tiny chemical entities that behave in such a way that one moment you can see them, and the next you cannot.
“In this work, our international team deployed enzyme kinetics, high-resolution X-ray synchrotron crystallography, advanced molecular dynamics and prediction tools, such as ancestral sequence reconstruction, to understand the roles of water molecules.
“This multidisciplinary approach allowed us to understand their evolutionary trajectories and formulate principles for water molecule dynamics in hydrolytic reactions and how water molecules form harmonised or non-random networks at atomic levels.”
Water is one of the smallest and most abundant molecules in the universe. It fulfils multiple metabolic roles as a solvent, substrate, cofactor, intermediate, and product, during biochemical transformations in living systems, such as plants and animals.
There are up to 80,000 enzymes fundamental to life that use water as a reactant, catalysing and speeding up biochemical reactions upon which almost all metabolic and physiological processes depend.
These processes include the hydrolysis of carbohydrate substrates such as cellulose, starch, and other glycosides during growth and development of all forms of life. This function allows enzymes, including plant hydrolases, to efficiently recycle polymeric substrates and support primary root extension, seed germination, and pollination.
In addition to the multibillion-dollar biomedical, pharmaceutical, food and chemical industries, this discovery could impact enzyme design and bioengineering, food, paper, pulp, bioplastics and textile materials processing, and biofuel production.
“Discoveries such as these are significant for product manufacturing through biotechnologies and foster the development of novel bioengineered hydrolytic enzymes,” Professor Hrmova said.
“These optimised enzymes could also function outside biological systems to produce pharmaceuticals, nutra-chemicals, drugs, chemicals, herbicides, pesticides, and other reagents.”
This study builds on prior foundational work by Professor Hrmova and her team in the School of Agriculture, Food and Wine and the Waite Research Institute.
“The interdisciplinarity of our work – integrating techniques, tools, concepts, and theories – has allowed us to resolve the enigma of these processes amongst complex research challenges.”
“In a broader context, here and other studies, we identified the operators regulating water flux and networks during hydrolytic reactions, which with other central phenomena such as processivity and reactant movements through trajectories, are all fundamental to catalysis,” said Professor Hrmova.
Animated visualisations describing the evolution of water networks in a plant exo-hydrolase are available here.



 Australia; VIC; SA
Australia; VIC; SA