News release
From:
Prodigious siblings can be annoying. All too often, they steal the spotlight and cast behind them an infuriating shadow of achievement and high expectation. The same is true in mineralogy, where some minerals have long existed in the shadow of their illustrious kin. For instance, when asked to name a phosphate mineral enriched in rare earth elements (REEs, lanthanum to lutetium, yttrium and scandium), most geoscientists will say monazite (REEPO4); a global superstar of geochronology, nuclear waste storage and critical rare earth element mineralization1,2,3 that forms across many hydrothermal, metamorphic and igneous environments. With 80% of the world’s rare earth elements — which are essential for the development of renewable technologies and for combating climate change — deriving from either monazite or its carbonate-rich cousin bastnäsite (REECO3F) (ref. 3), it is perhaps then unsurprising that monazite’s hydrous sibling rhabdophane (REEPO4 · xH2O, x = 0 – 1) gets so frequently overlooked.
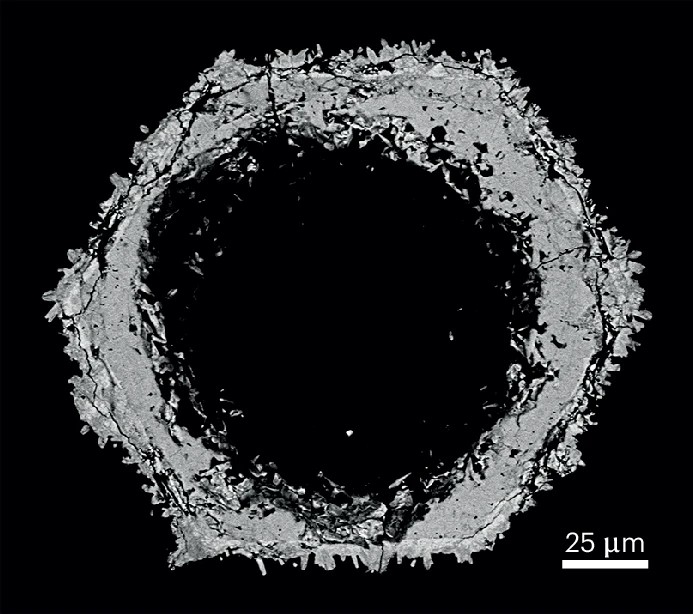
The origins of rhabdophane’s relative obscurity lie in its geological nature. Rhabdophane forms exclusively from low temperature (<250 °C) fluids4, which cause it to precipitate as a fine-grained, porous and analytically challenging mineral across a smaller diversity of rocks than monazite. Still, there are important rare earth-mineralizing systems where rhabdophane should be considered as the star of the show. For example, rhabdophane is more likely than monazite to precipitate during chemical weathering, such that it is the dominant authigenic REE-phosphate mineral in most regolith-hosted deposits5 that account for over 80% of global heavy rare earth element (europium to lutetium) resources3. Since mineralization of the rare earth elements in these deposits occurs via their easily leachable adsorption onto clay minerals3, their alternate capture by insoluble rhabdophane is detrimental to economic accumulation and is therefore essential to understand. Rare earth mineralization in other types of regolith deposit can also be mineral-hosted, like at the Mt Weld carbonatite where rhabdophane exists as an important source of critical metals6 despite the controls on its natural formation receiving little direct investigation until recently4,5.
Elsewhere, rhabdophane may also form during the low-temperature hydrothermal alteration of rare earth element-rich igneous intrusions like carbonatites and pegmatites (Fig. 1). In these instances, rhabdophane presents a useful tool to help understand the nature of associated rare earth element (re)mobilization or accumulation during alteration, since it intrinsically offers information as to the temperature of the hydrothermal fluid. For this information to be known, however, the rhabdophane must be accurately discerned from monazite4. In cases where it isn’t, the imprecise identification of rhabdophane relative to its overbearing anhydrous sibling may lead to critical rare earth element deposits being under-characterized or mischaracterized.
Since rhabdophane is a low temperature mineral, it also forms close to the Earth’s surface and impacts our daily lives. Rhabdophane governs contaminant transport and nutrient availability in soils7,8, forms in nuclear waste repositories as a phase that controls the solubility of the actinides1 — a group of radioactive elements — and is even found to have formed in human lungs after what is hoped was a single instance of two photographers inhaling rare-earth-oxide-rich lamp ash9.
Monazite’s under-appreciated sibling therefore deserves more attention. An improved understanding and identification of rhabdophane holds untold potential not only for the characterization of rare earth element deposits, but also for uranium-thorium-lead geochronometry that is underutilized in hydrothermal systems which are too cold for monazite to precipitate2. Sure, rhabdophane is often a fibrous, fine-grained and analytically challenging mess of a mineral4, but it is both fair and scientifically beneficial that rhabdophane be loved by way of rigorous investigation. After all, it is important that all rare earth minerals are considered.
The full published article Rare-earth--rich rhabdophane is published in Nature Geoscience, authored by Tobias G Bamforth, Sustainable Geochemistry and Mineral Sciences, School of Mathematics, Statistics, Chemistry and Physics, Murdoch University, Perth, Western Australia.
- Du Fou de Kerdaniel, E., Clavier, N., Dacheux, N., Terra, O. & Podor, R. J. Nucl. Mater. 362, 451–458 (2007).Article Google Scholar
- Rasmussen, B., Zi, J.-W. & Muhling, J. R. Geology 49, 1467–1472 (2021).Article CAS Google Scholar
- Li, Y. H. M., Zhao, W. W. & Zhou, M.-F. J. Asian Earth Sci. 148, 65–95 (2017).Article Google Scholar
- Bamforth, T. G. et al. Chem. Geol. 666, 122307 (2024).Article CAS Google Scholar
- Bamforth, T. G. et al. Miner. Depos. 59, 1479–1503 (2024).Article CAS Google Scholar
- Lottermoser, B. G. Lithos 24, 151–167 (1990).Article CAS Google Scholar
- Cervini-Silva, J., Fowle, D. A. & Banfield, J. Am. J. Sci. 305, 711–726 (2005).Article CAS Google Scholar
- Buriánek, D., Ivanov, M., Janderková, J. & Patzel, M. Catena 213, 106166 (2022).Article Google Scholar
- Sticher, H., Spycher, M. A. & Ruettner, J. R. Nature 241, 49 (1973).
Multimedia



 Australia; WA
Australia; WA