News release
From:
Neuroscience: Non-invasive spinal-cord stimulation improves arm and hand function
A non-invasive device for spinal-cord stimulation improved arm and hand function in 43 participants with tetraplegia (paralysis of the upper and lower body), according to results from a clinical trial published in Nature Medicine. The trial, which was completed by 60 participants, suggests that the therapy is safe and effective, meeting the primary endpoints of the trial.
Spinal-cord injuries affect the relationship between the brain and the spinal cord that regulates neurological functions, and when these occur in the cervical spine (neck area), they usually impair hand and arm function. Electrical stimulation of the spinal cord has been shown to restore impaired neurological functions when the stimulation is applied over the spinal segments that contain the neurons involved in the control of these functions. However, these approaches usually rely on invasive surgical procedures for the implantation of electrodes in specific regions of the spinal cord.
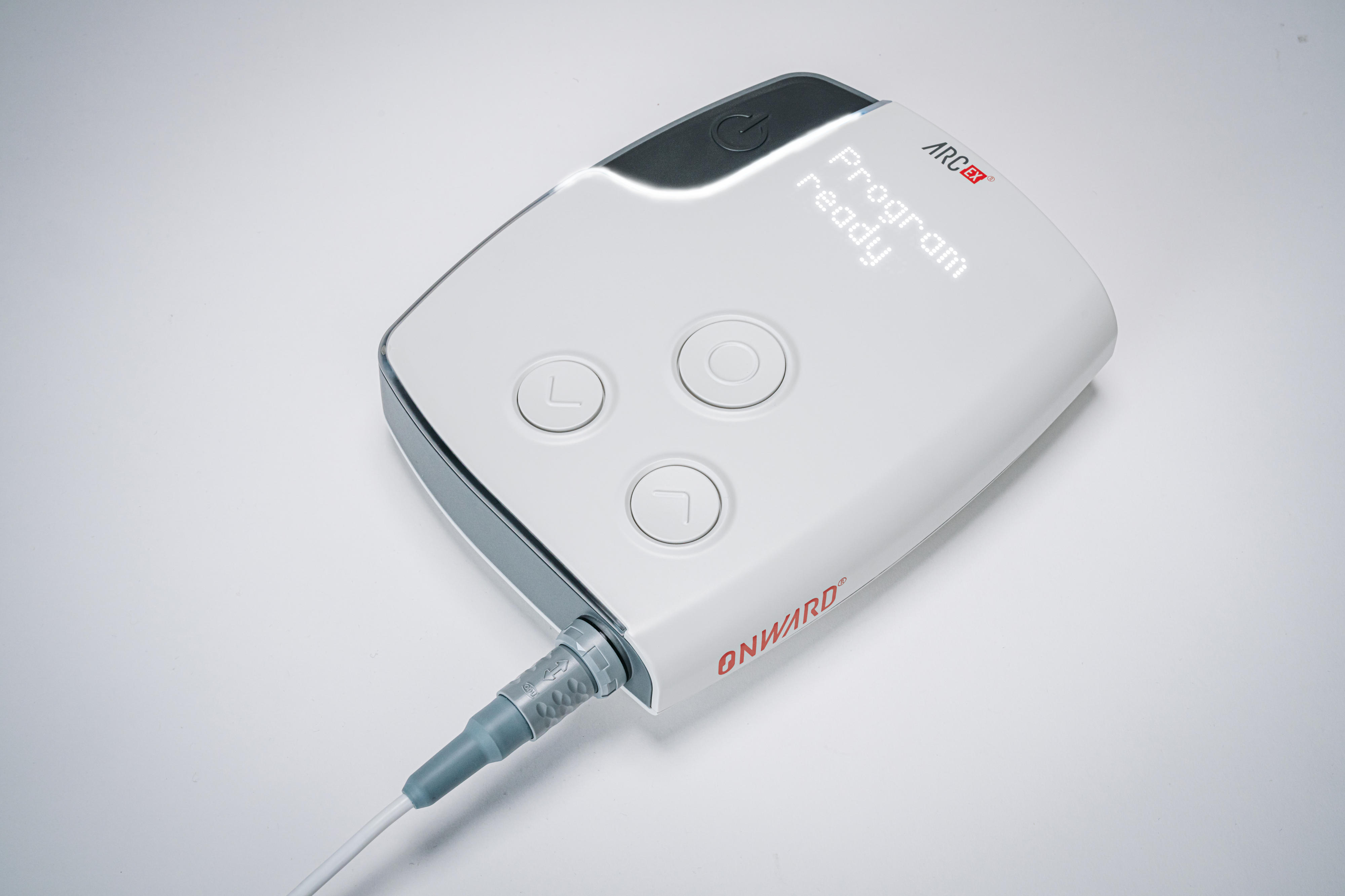
Gregoire Courtine and colleagues engineered a non-invasive device, called ARCEX, that delivers an electrical current to the spinal cord through surface electrodes that can then modulate neurons within the targeted spinal segments. To test the device’s effect on arm and hand function compared to the results of rehabilitation alone, the authors conducted a multi-center open-label clinical trial in 65 patients with tetraplegia as a result of a spinal-cord injury — only patients at 12 months or more after injury were included in this study. All participants enrolled in the clinical trial underwent a standardized in-clinic rehabilitation program over a period of 2 months, followed by the same rehabilitation program with the addition of ARCEX Therapy for an additional 2 months. There were no notable safety issues associated with ARCEX Therapy, and of the 60 participants who completed the trial, 43 demonstrated improvements in strength domains and functional domains. Secondary analysis revealed increases in fingertip pinch force, hand movement, and strength and sensory ability, as well as self-reported improvements in quality of life.
Courtine and colleagues note that their findings indicate that ARCEX Therapy is safe and effective and suggest it could serve as a new treatment to improve the neurological recovery of hand and arm function for people living with a chronic cervical spinal injury.
Please note that an online press briefing for this paper took place UNDER STRICT EMBARGO on Thursday 16th May at 3 pm London time (BST) / 10 am US Eastern Time.
Authors Gregoire Courtine, Chet Moritz and Edelle Field-Fote joined to discuss the research, alongside two patients who took part in the study. This was followed by a Q&A session.
A recording of the briefing is available
Multimedia





 International
International