News release
From:
Expert Reaction
These comments have been collated by the Science Media Centre to provide a variety of expert perspectives on this issue. Feel free to use these quotes in your stories. Views expressed are the personal opinions of the experts named. They do not represent the views of the SMC or any other organisation unless specifically stated.
Professor Adrian Esterman is Chair of Biostatistics at the University of South Australia
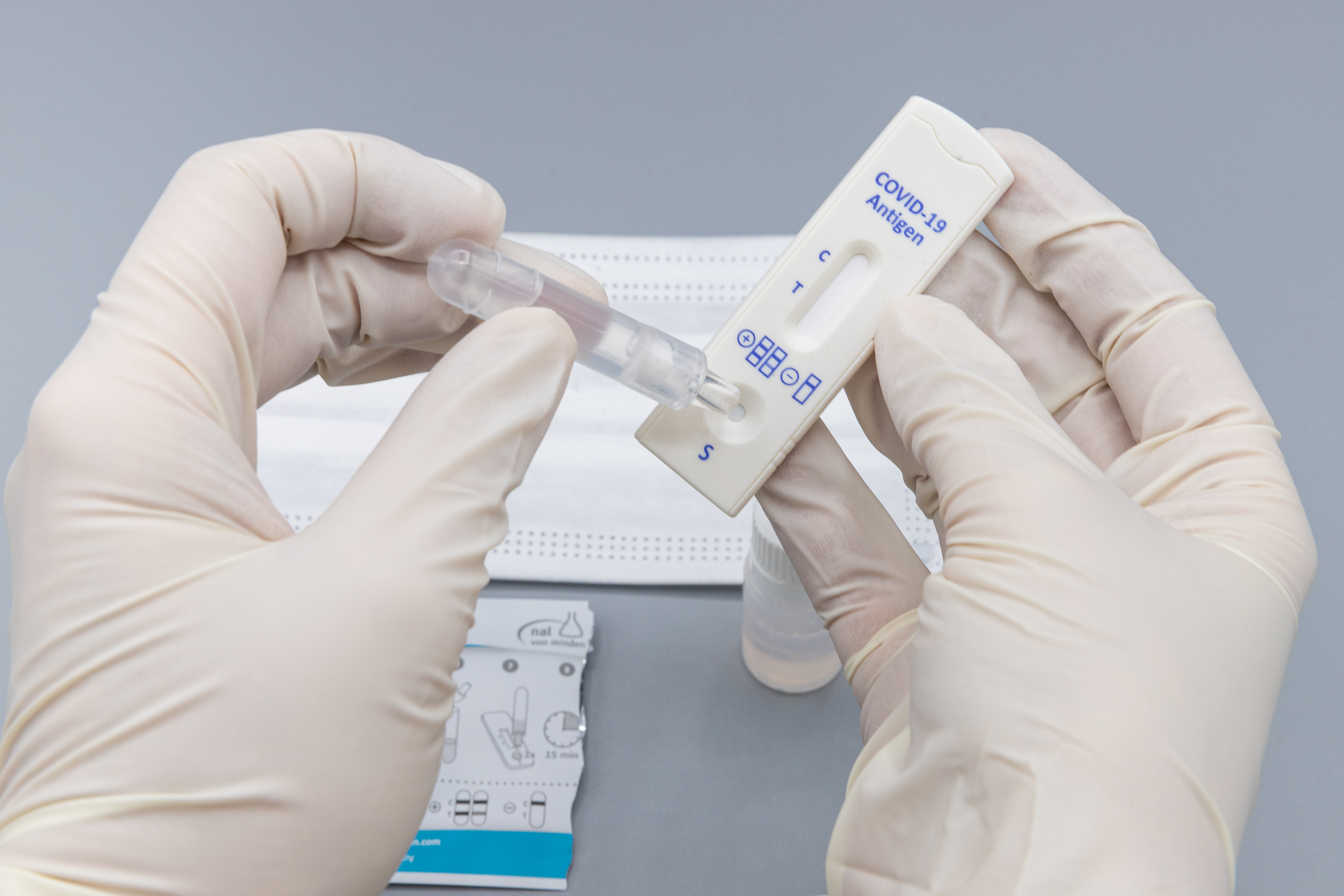
PCR tests work by looking for fragments of the virus in the swab sample. They require complicated analytic equipment only found in pathology laboratories and need expert lab technicians to run the equipment. Each PCR test takes about six hours to run, so it is not surprising that tests are returned usually after 24 hours. Rapid antigen tests are almost the opposite. They work by looking for proteins called antigens on the surface of the virus. They come in a test kit, a bit like the ones used for pregnancy, have results available in about 15 minutes, and are inexpensive.
In the UK, packets of ten test kits are available free of charge from chemist shops for home use. They are also provided free in several states in the USA. The TGA at the moment, only allow their use if supervised by a health professional, and there is some evidence that the results are a bit more accurate if this is done.
However, I really can’t see why there should be too much objection to people using them at home.
With respect to accuracy, they are not as good as the PCR test, and tend to produce more false positives and false negatives, especially when there is not much COVID about. However, if someone has a positive rapid antigen test result, then they should immediately go for a PCR test. Finally, rapid antigen tests work best when there is a high viral load, that is in the first two to three days before symptoms develop, and in the first week after symptoms.
Rapid antigen tests are ideal for airports, transport hubs, industry settings, schools, etc. Their slightly poorer accuracy is less important in these situations. They are not widely available in Australia, yet a popular one used in the USA is made in Queensland!
Troy Stewart is Managing Director of SureScreen Australia Pty Limited
A fast moving virus like the Delta strain needs a fast test. This allows rapid detection of virus leading to faster contact tracing and faster suppression of the virus. When used for repeat testing (at least every three days), rapid antigen tests (RATs) are as accurate as PCR and will allow people to safely work and return to their communities while limiting the risk of viral transmission.
Rapid antigen tests of suitable sensitivity, approved by the TGA, will have a role to play in the COVID-19 testing regimen deployed in Australia. These tests will best be deployed for high frequency testing – every three days - in at-risk and essential workplaces to rapidly detect and isolate infected individuals. Thus, keeping workers safe and the economy running smoothly.
The evidence is now very strong that repeat testing in at-risk cohorts and taking rapid action to isolate positive cases, is the best method of suppressing transmission.
Professor Thea van de Mortel is Deputy Head of the School of Nursing and Midwifery at Griffith University
Polymerase Chain Reaction (PCR) tests are the gold standard for diagnosing SARS-CoV-2 infection. They identify viral nucleic acids (like RNA and DNA). For a SARS-COV-2 infection the RT-PCR test uses a viral enzyme to convert coronavirus RNA to DNA and then uses viral nucleotide primers and a series of machine cycles to amplify the viral fragments to a detectable level, i.e. copying the fragments multiple times to make millions of copies, which are then detectable.
A rapid antigen test (RAT) detects an antigen - a viral protein capable of stimulating an immune response – by using chemicals to release the proteins and then putting the solution onto a paper strip that has the specific antibodies that bind to the antigen and create an observable change.
It can take hours to days to get a PCR test result, particularly when pathology labs are under pressure, whilst RATs can take as little as 15 minutes, depending on the brand. PCR can usually only be done in labs by trained technicians while the RAT can be done by anyone, anywhere, so can improve the speed and reduce the cost of getting a test result.
The downside of a RAT is that it has lower sensitivity (correctly detecting people who are infected) and specificity (correctly getting a negative result in an uninfected person).
The RAT is more prone to false positive (says you have a SARS-CoV-2 infection when you don’t) and false negative results (says you don’t have an infection when you do). The RAT is more likely to be accurate when you are symptomatic and viral load is high.
Jaya Dantas is Professor of International Health in the School of Population Health at Curtin University
Rapid antigen tests have been in use in several countries, for example, the Netherlands where it has been offered free since July 2021. The Dutch Government made available 3.5 million COVID-19 tests, both PCR and rapid antigen tests, to all Dutch nationals and travellers to the Netherlands through the Schengen Visa scheme travel companies.
These speedy tests typically mix nasal or throat swabs with liquid on a paper strip to return results.
Both tests are accurate – PCR tests results are available in 24 hours and rapid antigen tests in less than three hours, both in digital format. Whilst the global testing for SARS-CoV-2 relies on reverse transcription polymerase chain reaction (RT-PCR) tests performed on a nasopharyngeal specimen, this remains the gold standard for detecting SARS-CoV-2 as it is characterised by both high sensitivity and specificity in detecting viral ribonucleic acid (RNA).
Currently, the high volume of samples reaching laboratories leads to an increase in the turn-around time for PCR tests, sometimes due to a shortage of reagents and test kits.
Rapid antigen tests offer multiple benefits in comparison to RT-PCR tests. They can be used both in laboratory-based tests and for near-patient/client use (point-of-care), and results are normally generated in 10-30 minutes to three hours after the start of the analysis, and at low cost.
Most currently available rapid antigen tests show a lower sensitivity compared to the standard RT-PCR test, while their specificity is reported to be high. It is important to note that rapid antigen tests may be sensitive enough to detect cases with a high viral load, i.e. pre-symptomatic and early symptomatic cases (up to five days from symptom onset).
We know that other countries are opening borders for the vaccinated and using a number of strategies among their population to learn to live with the virus in a cautious and informed way. Antigen tests are easy to use and are currently being used in the USA, UK, Singapore and many European countries. Due to their quick turnaround testing times, they can play a part with truck drivers, sports people, teachers, travellers and the general population just wanting to know their COVID-19 status.
Professor Paul Griffin is the Director of Infectious Diseases at Mater Health Services and the Head of the Mater Clinical Unit for the University of Queensland School of Medicine
In an effort to maximise our control of COVID-19 we need to utilise all available tools in our arsenal. This should definitely include rapid antigen tests. However, like all tests, they need to be used in the right way and their results interpreted in the correct context. A large part of our success in this country has been the widespread access to highly accurate PCR based testing, often with relatively short turn around times compared to many other parts of the world.
Rapid antigen testing will not replace the use of these laboratory-based PCR tests, but I see it as something that could be used in finite circumstances, in a complementary fashion. While clearly the shorter turnaround time and ability to perform at home or in your workplace are useful benefits, rapid antigen tests do not have the same level of accuracy as PCR based testing. A false negative in someone who was potentially infectious, if they were not mindful of the limitations of this type of testing, could have very significant consequences in terms of contributing to the onward transmission of the virus.
The Royal College of Pathologists of Australasia are the leading professional organisation representing pathologists, medical specialists and scientists responsible for pathology testing in Australia and they have released a very useful statement on the 10th of August that I would encourage people to be aware of as it includes an explanation of some of these limitations.



 Australia; NSW; QLD; SA; WA
Australia; NSW; QLD; SA; WA