News release
From:
Engineering molecules to turn off inflammation
In a long-term collaboration between researchers and industry, an exciting first step has been made in the creation of a new generation of medicines for auto-immune diseases such as arthritis and inflammatory bowel disease using one of our body’s own anti-inflammatory off-switch molecules.
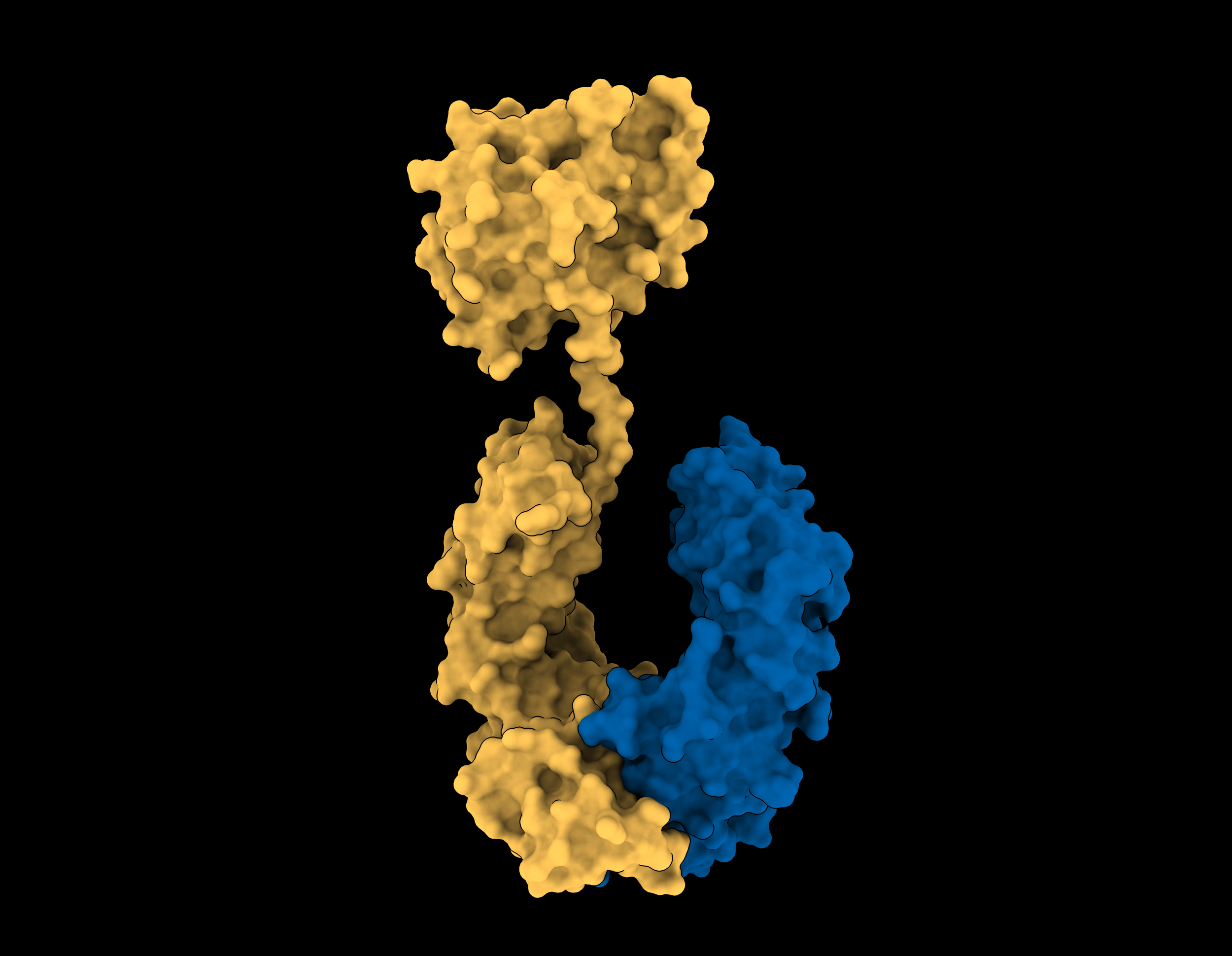
Our body’s own immune system produces many highly potent anti-inflammatory molecules, but they are often highly fragile, short-lived, and do not have drug-like properties. Interleukin-37 is one such molecule produced by the body to turn off inflammation.
Together with partner F. Hoffmann-La Roche (Roche), the multidisciplinary research team from the Monash University Biomedicine Discovery Institute (BDI), Monash University’s Department of Paediatrics and the Hudson Institute of Medical Research have harnessed their Fc-fusion platform to engineer the next generation of Interleukin-37, one that retains anti-inflammatory potency, is highly stable and has an excellent therapeutic likeness.
The findings from the research collaboration have now been published in Cell Chemical Biology.
A little bit of inflammation can be a good thing and is often the body's immune system doing its job. However, when inflammation persists, or the immune system starts attacking the body’s own cells, this can lead to disease.
One of the study’s lead authors Dr Andrew Ellisdon from the Monash BDI, says many human diseases, including autoimmune diseases such as arthritis or inflammatory bowel disease, are characterised by too much inflammation.
Dr Ellisdon says there has been a gap in producing new generations of potent anti-inflammatory therapeutics for these anti-inflammatory conditions.
“The past five years have been a remarkable opportunity to collaborate and learn from Roche, a global leader in the engineering and development of biologics. Because of the data from this study, we now know how to make more stable and medicine-like versions of the body’s own anti-inflammatory off-switches,” he said.
“This study builds a solid platform to test IL-37-based Fc-fusion variants in a range of preclinical models of inflammatory and autoimmune disease. We anticipate that many of the steps undertaken in this Fc-engineering platform will be broadly applicable to other challenging and unstable biologics.”
Professor Marcel Nold, a Professor for Paediatric Immunology at Monash University and a consultant Neonatal Paediatrician at Monash Children’s Hospital, said:“It is a clinician scientist's ultimate goal to be part of research that can be translated into future patient treatments.”
Associate Professor Claudia Nold at the Hudson Institute of Medical Research and one of the lead scientists, said: “Working in the field of inflammation, we were delighted to partner with Roche, a Swiss pioneer in healthcare since 1896. Our collaboration between academia, clinicians and industry partners such as Roche enabled us to leverage our combined expertise and solve biomedical questions to get a step closer in developing innovative medicines for patients suffering from inflammatory diseases.”
Read the full paper in Cell Chemical Biology titled: Protein engineering of a stable and potent anti-inflammatory IL-37-Fc fusion with enhanced therapeutic potential.
DOI: 10.1016/j.chembiol.2021.10.004



 Australia; VIC
Australia; VIC