News release
From:
JAMA
Safety Surveillance of COVID-19 mRNA Vaccines Through the Vaccine Safety Datalink
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, SEPTEMBER 3, 2021
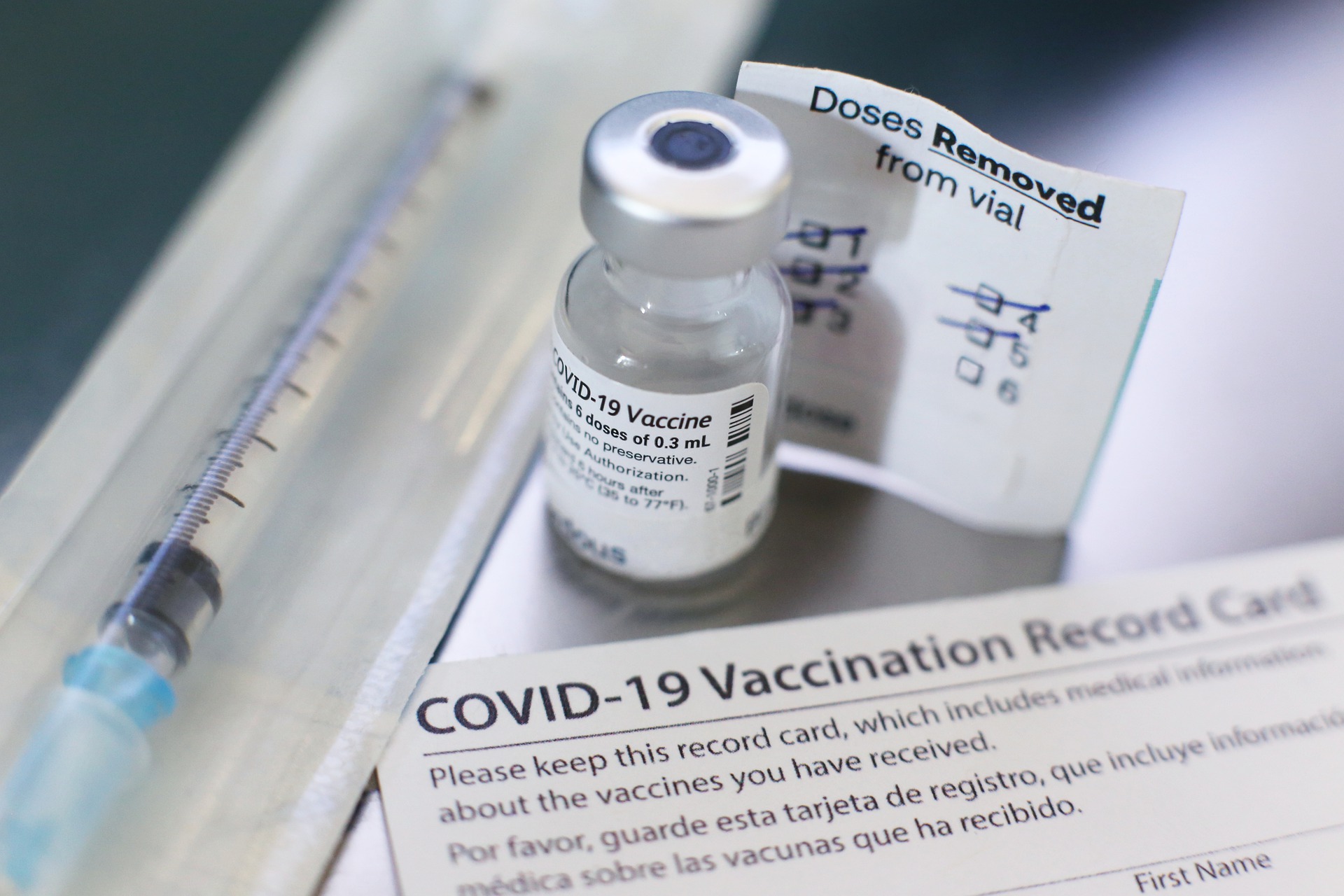
What The Study Did: In this interim analysis of surveillance data from 6.2 million people who received 11.8 million doses of an mRNA vaccine, event rates for 23 serious health outcomes weren’t significantly higher for individuals one to 21 days after vaccination compared with similar individuals at 22 to 42 days after vaccination.
Attachments
Note: Not all attachments are visible to the general public.
Research URLs will go live after the embargo ends.

Research
JAMA, Web page
The URL will go live after the embargo ends.
Organisation/s:
Kaiser Permanente Northern California, USA
Funder:
This study was supported by grant funding from the CDC, contract 200-2012-53581/0001. Dr Klein reported receiving grants from the Centers for Disease Control and Prevention (CDC) during the conduct of the study, and grants from Pfizer, Merck, GSK, Sanofi Pasteur, and Protein Science (now SP) outside the submitted work. Ms Hanson reported receiving grants from CDC during the conduct of the study. Dr Donahue reported receiving grants from CDC during the conduct of the study and from Janssen Vaccines & Prevention for a study unrelated to COVID-19 vaccines. Dr Kharbanda reported receiving other from CDC (contract 200-2012-53526) during the conduct of the study



 International
International