News release
From:
Springer Nature
Respiratory infections may increase breast cancer lung metastases
Respiratory viruses trigger metastatic breast cancer cells to proliferate in lungs in mouse models of breast cancer, a study in Nature indicates. These findings are supported by human observational data. The work sheds light on the association between infectious diseases and cancer metastasis.
Breast cancer is the most diagnosed cancer in women. After an initial remission, cancer cells may remain dormant for years before metastasis (in the lungs or other organs) causes a relapse. Viral respiratory infections, such as SARS-CoV-2, are associated with inflammation, which may trigger processes that could influence metastasis.
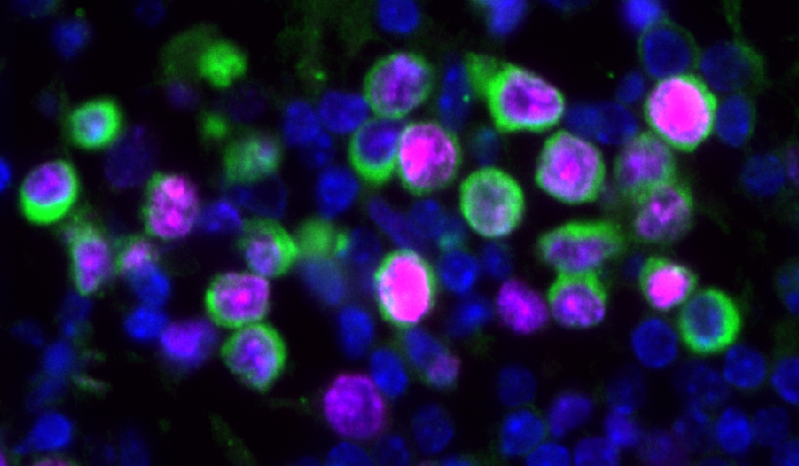
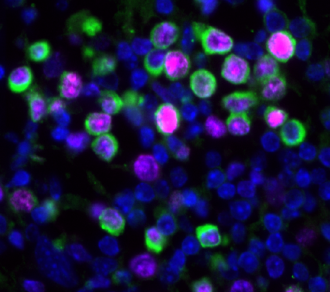
An increase in cancer death rates in the first two years of the COVID-19 pandemic prompted James DeGregori and colleagues to study the effects of influenza virus and SARS-CoV-2 infections on breast cancer outcomes in mouse models. They found that these infections reduced the dormancy of breast cancer cells in the lungs. These cells proliferated within days of infection and led to the expansion of metastatic cancer lesions within two weeks. The authors find that inflammatory pathways are implicated in this effect.
The authors also examined whether patients with cancer that tested positive for SARS-CoV-2 might have an increased risk of cancer-related death. They analysed data from the UK Biobank (4,837 participants, all cancer types) and Flatiron Health (36,845 patients with breast cancer) databases. There was an association between SARS-CoV-2 infection and risk of death in the Biobank group; patients that tested positive versus negative for SARS-CoV-2 showed a twofold increase in cancer-related death. In the Flatiron group, SARS-CoV-2 infection was associated with an increase of over 40% in the risk of metastatic disease in the lungs.
Together, the findings reveal how respiratory virus infections may increase the risk of cancer relapse and highlight the need for strategies to address the increased risk of metastatic progression associated with respiratory virus infections, the authors conclude.
Attachments
Note: Not all attachments are visible to the general public.
Research URLs will go live after the embargo ends.

Research
Springer Nature, Web page
The URL will go live after the embargo ends
Journal/
conference:
Nature
Organisation/s:
University of Colorado, USA
Funder:
We acknowledge funding from grant 1 I01 BX004495 from the
Department of Veterans Affairs to J.D.; the Courtenay C. and Lucy Patten Davis Endowed Chair
in Lung Cancer Research to J.D.; the Kay Sutherland and Monika Weber Research Fund to J.D.;
and an Across the Finish Line grant from the University of Colorado School of Medicine to J.D.,
M.R. and T.E.M. M.R. is supported by a National Institutes of Health (NIH)/National Cancer
Institute (NCI) award R01CA260909. J.D., M.R. and J.A.A.-G. are supported by NIH/NCI award
R01CA301643. J.M.B. is supported by R01AG081226, R01AI173305 and P30 AG067988. This
work was also supported by NIH/NCI awards R01CA109182 and P30CA013330, the Samuel
Waxman Cancer Research Foundation Tumor Dormancy Program to J.A.A.-G., who is also a
Samuel Waxman Cancer Research Foundation Investigator. S.B.C. was supported by grant
AWD-232405-SC from the Cancer League of Colorado. B.J.J. was supported by T32 NIGMS/NIH
5T32GM141742-02. A.N.C. is supported by a NIGMS/NIH predoctoral fellowship (T32AR079114).
F.G. acknowledges funding from the MRC Centre for Global Infectious Disease Analysis
(reference MR/X020258/1), funded by the UK Medical Research Council (MRC); this UK-funded
award is carried out in the frame of the Global Health EDCTP3 Joint Undertaking. H.M. is
supported by the UK National Institute for Health Research’s Comprehensive Biomedical
Centre at University College London Hospitals. M.P. was funded by the Cancer Research
Institute Irvington Postdoctoral Fellowship (CRI5290). D.C.W. was supported by NIH grants
R01CA259635 and R01AG078814, DOD grant W81XWH-21-1-0128 and the Bill & Melinda Gates
Foundation grant INV-046722. We thank the Rocky Mountain Regional VAMC Flow Core; the
Biostatistics and Bioinformatics, Genomics, Human Immune Monitoring, and Flow Cytometry
Shared Resources supported by National Cancer Institute grant P30CA046934 to the
University of Colorado Cancer Center; and the Gates Institute Histology Core at the University
of Colorado. This work was supported by the Alpine HPC system, which is jointly funded by the
University of Colorado Boulder, the University of Colorado Anschutz, Colorado State University
and the National Science Foundation (award 2201538). We thank G. Geno, W. Wang and
M. Wang and J. Figura for guiding our studies with the Flatiron Health database; C. Swanton for
connecting us with the team that did the UK Biobank analyses; and D. Merrick and P. Jedlicka
for advice on the evaluation of DCC pathology.




 International
International