News release
From:
Peer-reviewed Experimental study Cells
Bite-sized chunks of chicken with the texture of whole meat can be grown in the lab
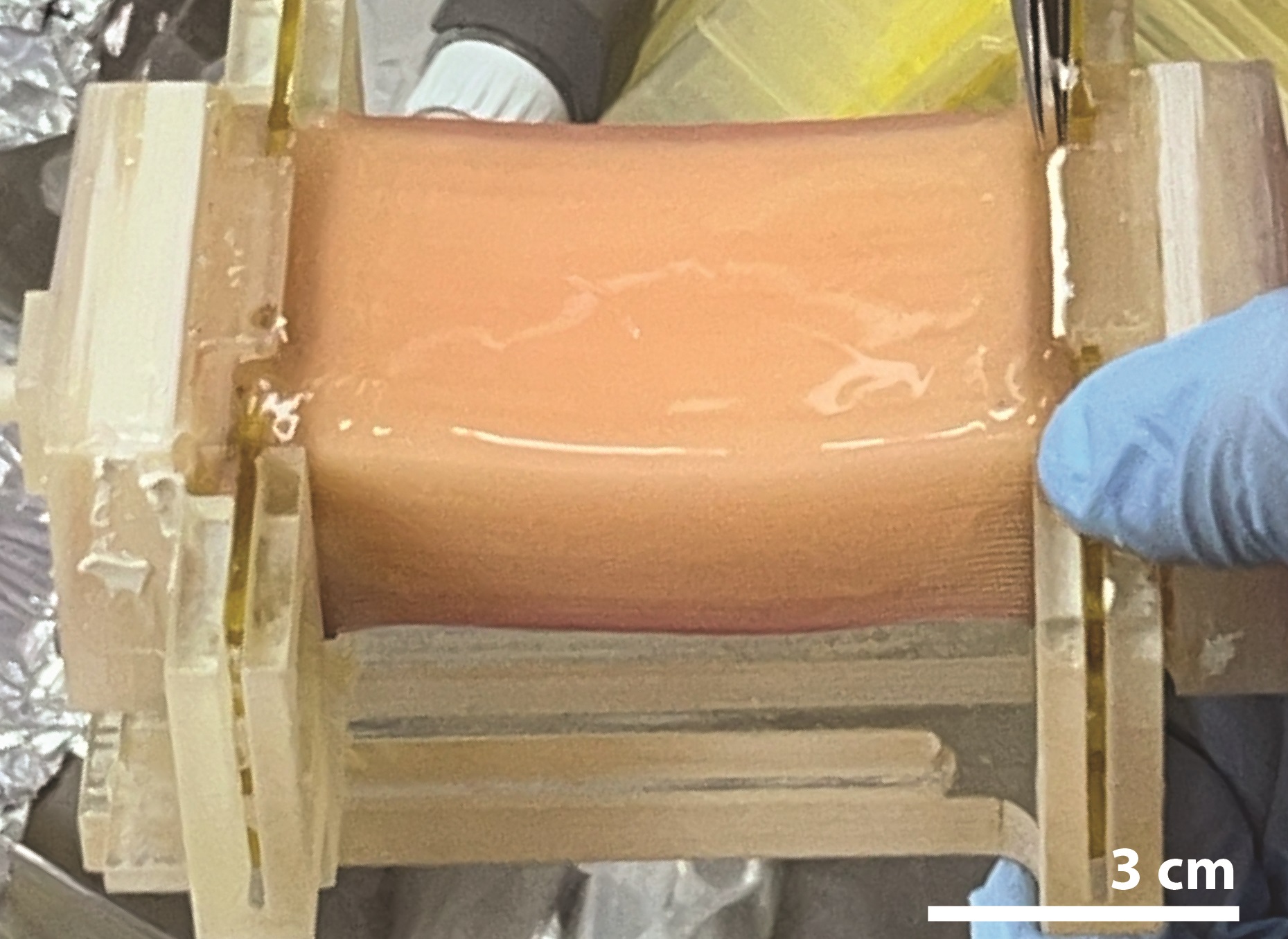
A bioreactor that mimics a circulatory system can deliver nutrients and oxygen to artificial tissue, enabling the production of over 10 grams of chicken muscle for cultured meat applications. These results are publishing in the Cell Press journal Trends in Biotechnology on April 16.
“Our study presents a scalable, top-down strategy for producing whole-cut cultured meat using a perfusable hollow fiber bioreactor,” says senior author Shoji Takeuchi of The University of Tokyo. “This system enables cell distribution, alignment, contractility, and improved food-related properties. It offers a practical alternative to vascular-based methods and may impact not only food production but also regenerative medicine, drug testing, and biohybrid robotics.”
A significant obstacle to the reconstruction of large-scale tissues is the creation of well-distributed vascular networks because diffusion alone cannot sustain cells across considerable distances. The thickness of tissues without an integrated circulatory system has generally been limited to less than 1 mm, making it challenging to produce centimeter-scale or larger tissues with densely packed cells.
“We're using semipermeable hollow fibers, which mimic blood vessels in their ability to deliver nutrients to the tissues,” Takeuchi says. “These fibers are already commonly used in household water filters and dialysis machines for patients with kidney disease. It's exciting to discover that these tiny fibers can also effectively help create artificial tissues and, possibly, whole organs in the future.”
The authors demonstrated the biofabrication of centimeter-scale chicken skeletal muscle tissues using a Hollow Fiber Bioreactor (HFB) consisting of an array of 50 hollow fibers. In addition, they implemented a robot-assisted assembly system for the fabrication of a 1,125-fiber HFB and produced whole-cut chicken meat weighing more than 10 g using chicken fibroblast cells, which make up connective tissue.
“Cultured meat offers a sustainable, ethical alternative to conventional meat,” Takeuchi says. “However, replicating the texture and taste of whole-cut meat remains difficult. Our technology enables the production of structured meat with improved texture and flavor, potentially accelerating its commercial viability. Beyond food, this platform may also impact regenerative medicine and soft robotics.”
According to Takeuchi, additional challenges for future research include determining the long-term effects of perfusion on tissue quality, adapting the technology for organ fabrication and biohybrid robotics, and further improving the mechanical properties and structural integrity of the tissue to better mimic the characteristics of natural muscle tissue.
“We overcame the challenge of achieving perfusion across thick tissues by arranging hollow fibers with microscale precision,” Takeuchi says. “Remaining challenges include improving oxygen delivery in larger tissues, automating fiber removal, and transitioning to food-safe materials. Solutions may include use of artificial oxygen carriers to mimic red blood cells, bundle-removal mechanisms that efficiently remove fibers in a single operation, and edible or recyclable hollow fibers.”



 International
International