Media release
From:
WEHI researchers have visualised a key protein complex in malaria parasites for the first time, uncovering a new target for next-generation vaccines that could help stop the disease from spreading.
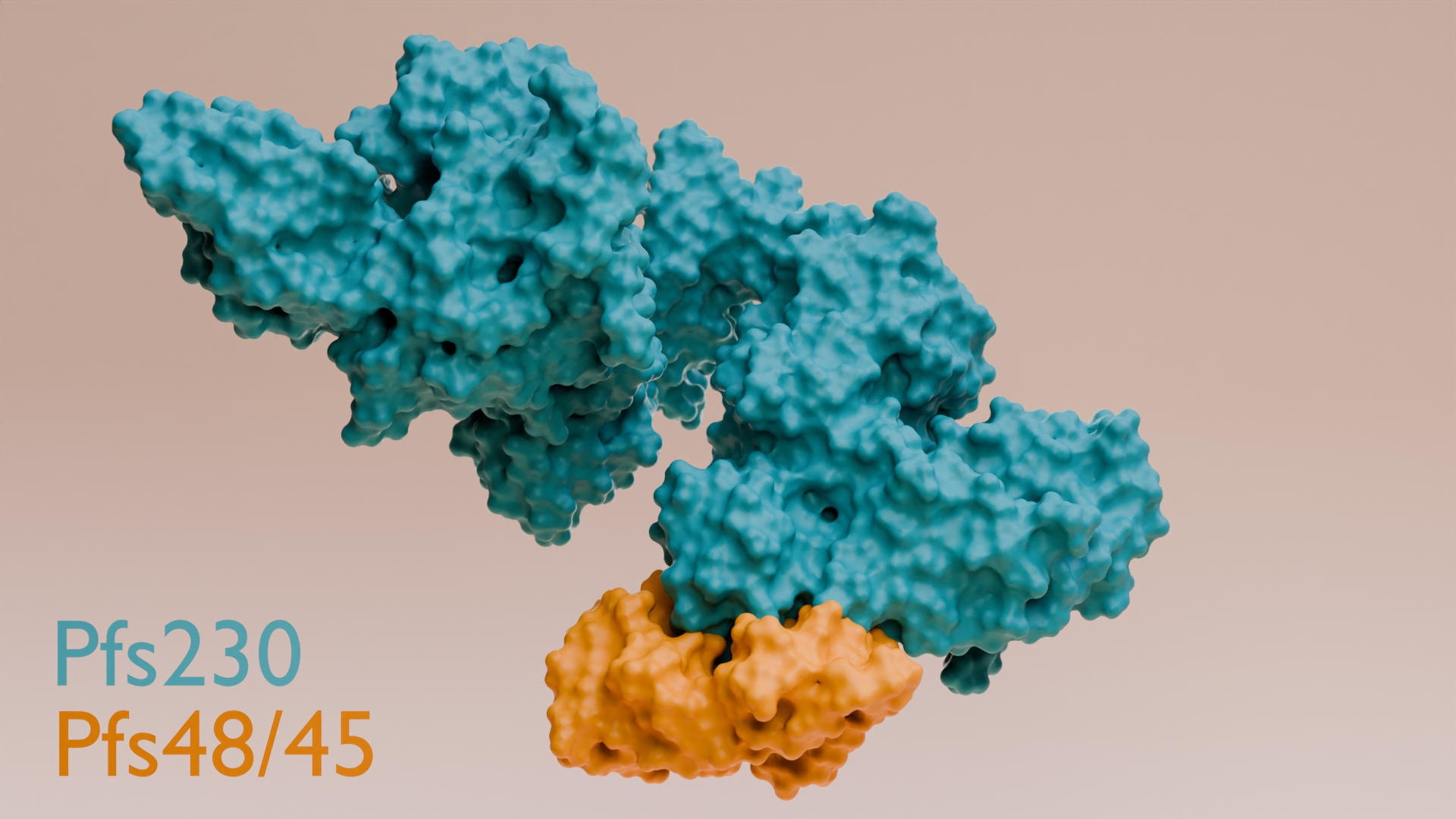
Using cutting-edge cryo-electron microscopy, the team captured the first detailed structure of a protein complex essential for malaria parasite fertilisation.
The discovery published in Science has led to the development of a promising new mRNA vaccine candidate that stops the malaria parasite from reproducing inside mosquitoes, breaking the cycle of transmission before it can reach humans.
Malaria remains one of the world’s deadliest infectious diseases, responsible for more than 600,000 deaths each year.
At a glance
*WEHI scientists captured the first high-resolution structure of a key protein complex that’s essential for the malaria parasite to reproduce inside mosquitoes.
*They discovered two small domains of the Pfs230-Pfs48/45 fertilisation complex that are crucial for the parasite’s ability to fertilise and spread.
*A new mRNA vaccine induced antibodies targeting these domains which stopped the parasite from reproducing in mosquitoes, cutting transmission by up to 99.7%.
Visualising malaria’s reproductive machinery
For many years scientists have known that two key proteins that appear on the surface of the malaria parasite, Pfs230 and Pfs48/45, are important for transmission of the disease.
Lead researcher Dr Melanie Dietrich said the new study revealed for the first time how these proteins interact – revealing a new vaccine target.
“Our structural biology approach was the key. Using cryo-electron microscopy, we were able to visualise the full fertilisation complex directly from the parasite – not a lab-made version,” Dr Dietrich, a WEHI postdoctoral fellow said.
"This gave us a clear picture of how this fertilisation complex really looks in nature, and revealed a previously unknown region that’s crucial to the process, unlocking a powerful new vaccine target.”
Lead researcher Professor Wai-Hong Tham said that by capturing the fertilisation complex directly from the parasite, the team revealed the precise contact points that make transmission possible.
"We used these findings to develop a vaccine that showed great promise in targeting these contact points,” Prof Tham, a WEHI laboratory head, said.
“To eliminate malaria, we need to stop transmission. This vaccine candidate could be one piece of that puzzle.”
From structural insight to vaccine innovation
Unlike many structural biology studies that rely on proteins made in the lab from bacterial, insect or mammalian cells, the new research successfully purified the fertilisation complex directly from malaria parasites – a technically challenging approach that ensures the structure reflects its true biological form.
The research revealed the critical contact points for binding the Pfs230 and Pfs48/45 proteins. When these were removed in genetically modified parasites, fertilisation failed and transmission was blocked, illuminating a new vaccine target.
Building on the structural discovery, the team designed a next generation mRNA vaccine which was formulated in collaboration with the mRNA Core facility at the Monash Institute of Pharmaceutical Sciences (MIPS).
In preclinical studies, the vaccine triggered high levels of antibodies that recognised the parasite and blocked transmission in mosquitoes by up to 99.7%.
Professor Colin Pouton from MIPS said it was an exciting opportunity for his team to leverage their expertise in mRNA vaccine development to address an important new target for malaria vaccination.
“Drawing on experience through mRNA Core, the MIPS team shifted focus to tackle a new challenge in malaria vaccination,” Prof Pouton said.
“The success of the malaria vaccine program illustrates the versatility of mRNA technology, which has many applications beyond the COVID vaccines. It was particularly rewarding to work on this project with the WEHI team, co-located in the Parkville precinct, where shared expertise has helped drive a new approach to malaria prevention."
A vulnerable stage in the parasite’s life cycle
Targeting the parasite inside the mosquito offers a strategic advantage due to what researchers call a population bottleneck.
While malaria parasites are abundant in the human host, only a small fraction develop into sexual forms and are successfully fertilised inside the mosquito. This bottleneck means that even modest reductions in parasite numbers at this stage can have a significant impact on transmission.
Transmission-blocking vaccines – like the one designed through this research, targeting the malaria parasite inside the mosquito – offer a strategic way to halt the spread of malaria, where its numbers are lowest and its life cycle most vulnerable.
Multi-stage strategy towards elimination
The team envisions the mRNA vaccine as part of a multi-stage strategy, targeting the parasite in both the mosquito and human host.
By combining transmission-blocking vaccines with those that act on blood or liver stages in people, researchers hope to build a comprehensive defence that could dramatically reduce malaria burden and move closer to elimination.
Prof Tham said the collaboration between WEHI and MIPS highlighted the strength of the Melbourne Biomedical Precinct and the potential of mRNA technology to rapidly translate basic science into vaccine innovation.
“The ability to design, formulate and test vaccine candidates within a single research ecosystem has accelerated the path from discovery to preclinical validation,” she said.
The study “Cryo-EM structure of endogenous Plasmodium falciparum Pfs230 and Pfs48/45 fertilization complex”, is published in Science (DOI: 10.1126/science.ady0241).
Multimedia



 Australia; International; VIC
Australia; International; VIC