News release
From:
A team at the Garvan Institute of Medical Research has discovered a group of brain cells that boosts appetite when there is a prolonged surplus of energy in the body, such as excess fat accumulation in obesity.
The researchers discovered that these cells not only produced the appetite-stimulating molecule NPY, but they in fact made the brain more sensitive to the molecule, boosting appetite even more.
“These cells kickstart changes in the brain that make it more sensitive to even low levels of NPY when there is a surplus of energy in the body in the form of excess fat – driving appetite during obesity,” explains Professor Herbert Herzog, senior author of the study and Visiting Scientist at Garvan.
“Our study addresses a long-standing question about how appetite is controlled in obesity and has the potential to take the development of therapy into a new direction.”
The research was published in the journal Cell Metabolism.
The discovery of a vicious cycle
Obesity is a major public health issue and a disease that affects more than one in 10 adults and increases a person’s risk of developing other chronic conditions, such as diabetes or heart disease. While many factors can influence the development of obesity – an excessive accumulation of fat tissue in the body – eating patterns and physical activity levels are key contributors.
“Our brain has intricate mechanisms that sense how much energy is stored in our body and adjust our appetite accordingly. One way it does this is through the molecule NPY, which the brain produces naturally in response to stresses, such as hunger, to stimulate eating,” says Professor Herzog.
“When the energy we consume falls short of the energy we spend, our brain produces higher levels of NPY. When our energy intake exceeds our expenditure, NPY levels drop and we feel less hungry. However, when there is a prolonged energy surplus, such as excess body fat in obesity, NPY continues to drive appetite even at low levels. We wanted to understand why.”
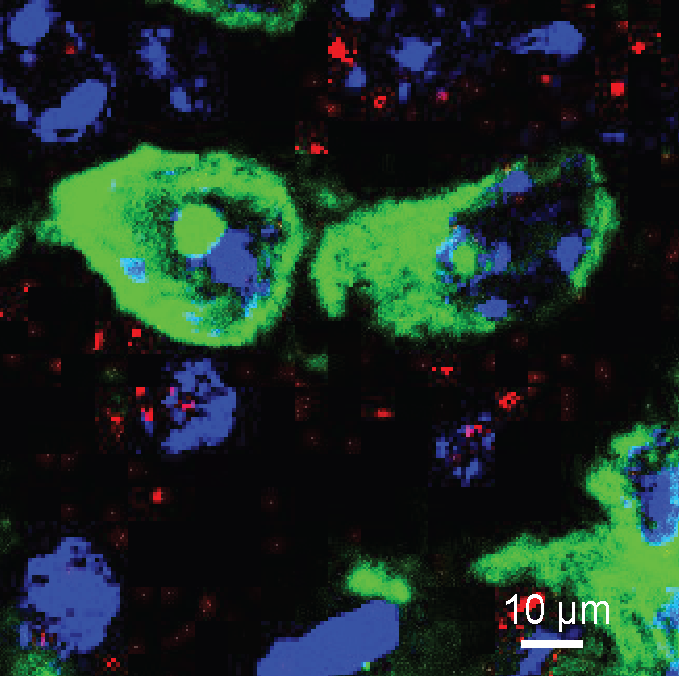
In mouse models of obesity, the researchers investigated different types of cells in the brain called neurons that produced NPY and discovered that surprisingly, 15% of them were different – they did not shut down NPY production during obesity.
“We found that under obese conditions, appetite was mostly driven by NPY produced by this subset of neurons. These cells did not only produce NPY, but also sensitised other parts of the brain to produce additional receptors or ‘docking stations’ for the molecule – supercharging appetite even further,” says Professor Herzog.
“What we have uncovered is a vicious cycle that disrupts the body’s ability to balance its energy input with energy storage and enhances obesity development.”
Wired to resist weight loss
“Our brain is wired to resist energy deficiency or weight loss, as it sees this as a threat to our survival and kickstarts mechanisms that increase our appetite so that we seek out food. As we found now, this even occurs when we have excess energy stored in the body,” Professor Herzog explains.
The researchers say their discovery opens the possibility of blocking the additional, more sensitised receptors for NPY as a new approach to developing anti-obesity medication.
“Our discovery helps us better understand the mechanisms in the brain that interfere with a balanced energy metabolism and how they may be targeted to improve health,” says Professor Herzog.
--ENDS--
This research was supported by the National Health and Medical Research Council (project grant 1144819). Professor Herzog is a Conjoint Professor at St Vincent's Clinical School, Faculty of Medicine and Health, UNSW Sydney.



 Australia; NSW
Australia; NSW