Media release
From:
Materials: Ocean-captured carbon converted into biodegradable plastic
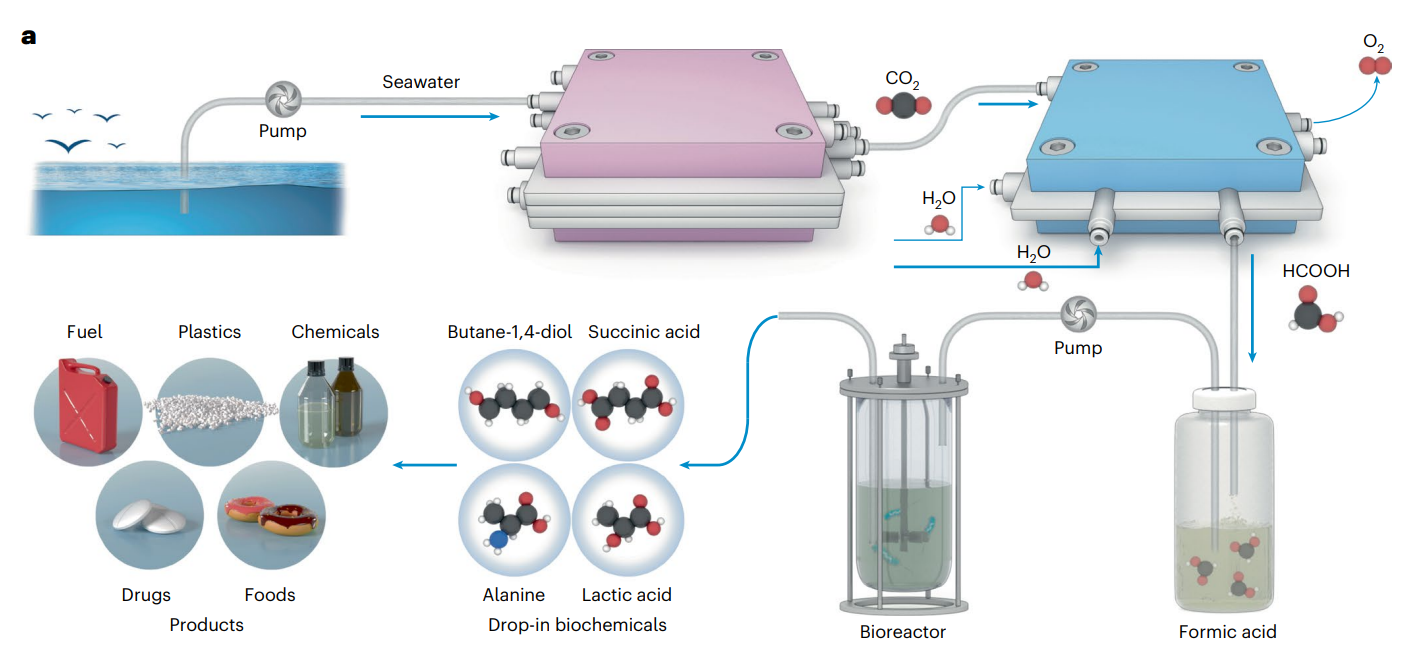
A system to capture carbon dioxide (CO₂) from seawater and convert it into biodegradable plastic precursors is reported in Nature Catalysis. The findings suggest a potentially sustainable way to produce industrial chemicals.
The ocean is the Earth’s largest carbon sink, absorbing about 25% of the CO₂ released by human activities. However, this uptake contributes to ocean acidification and risks destabilising marine ecosystems. Utilising this carbon resource presents a sustainable alternative to fossil fuels to produce important chemicals and materials, such as plastics.
Chuan Xia and colleagues engineered a two-part system that captures CO₂ from natural seawater with over 70% efficiency and low energy consumption (around 3 kilowatt-hours per kilogram of CO₂), operating continuously for 536 hours. The carbon capture cost was found to be competitive against current technology, at US$229.9 per tonne of CO₂. First, the CO₂ was converted into pure formic acid using an electrocatalyst. This was then transformed by engineered bacteria, Vibrio natriegens, into succinic acid, which is the starting material needed to prepare poly(butylene succinate), a biodegradable thermoplastic polymer. The researchers achieved production levels of up to 1.37 grams per litre in scaled-up fermenters.
The system could also be used to produce numerous other chemicals from CO₂ — for potential use in various products such as fuels, drugs and foods — by further engineering the catalysts used in each part (the electrode and the microorganism), the authors suggest. Although the system shows scalability and stability, further optimisation is needed to improve yields and integration for industrial use.
Multimedia



 International
International