Media release
From:
Medicine: Improving the compatibility of pig organs for transplantation into humans *PRESS BRIEFING* (N&V)
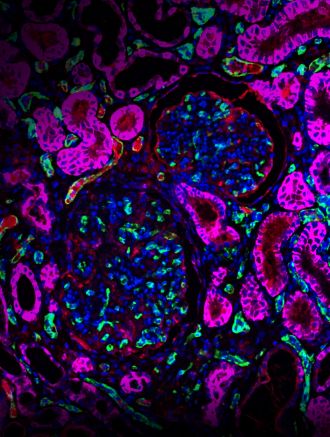
The design and successful transplantation of kidney grafts from genetically modified pigs into non-human primates is described in a study published in Nature. Modifying the pig genomes to remove antigen coding genes, add human genes and eliminate pig viruses, resulted in long-term survival of the monkey recipients, up to around two years. This preclinical work may move the field a step closer to clinical testing of genetically modified pig kidneys for human transplantation.
The transplantation of animal organs into humans (xenotransplantation) may offer a solution to the worldwide organ shortage. Pigs are promising donor animals but several obstacles first require overcoming before they can be considered clinically viable, notably organ rejection and risk of zoonosis (transmission of animal viruses to humans). Previous work has identified three glycan antigens expressed in pigs that are recognised by human antibodies and attacked, leading to rejection of the organ. The porcine endogenous retrovirus has also been identified as a risk for transmission into humans.
Wenning Qin and colleagues build on this previous research by introducing alterations into the genome of a donor pig and achieve successful transplantation of kidney grafts from a genetically engineered pig into a cynomolgus monkey model (a non-human primate with several human-like traits). The researchers introduced 69 genomic edits into the porcine donor (a Yucatan miniature pig), knocking out three glycan antigens thought to induce rejection, overexpressing seven human transgenes (to reduce hostility of primate immune system) and inactivating all copies of the porcine retrovirus gene. These kidney grafts survived substantially longer than grafts with only the glycan antigen knockouts (176 days versus 24 days), suggesting that the expression of these human transgenes offers some protection against rejection. Combined with immunosuppressive treatment, the transplant provided long-term primate survival of up to 758 days. These results demonstrate the promise of pig organs in future human transplantations and bring the technique a step closer to clinical testing, the authors conclude.
***
Please note that an online press briefing for this paper will take place UNDER STRICT EMBARGO on Tuesday 10 October at 3 pm London time (BST) / 10 am US Eastern Time**
Multimedia



 International
International



