Media release
From:
Scientists at La Trobe University have discovered a previously unknown way viruses could spread around the body, potentially paving the way for more effective drug development.
The research, published in Nature Communications, uncovers new understanding of the process of cell death and renewal.
Led by PhD candidate Stephanie Rutter in Professor Ivan Poon’s lab at the La Trobe Institute for Molecular Science (LIMS), the research shows how each step in the process is critical to help a dying cell break down and be cleared away by the body’s immune system.
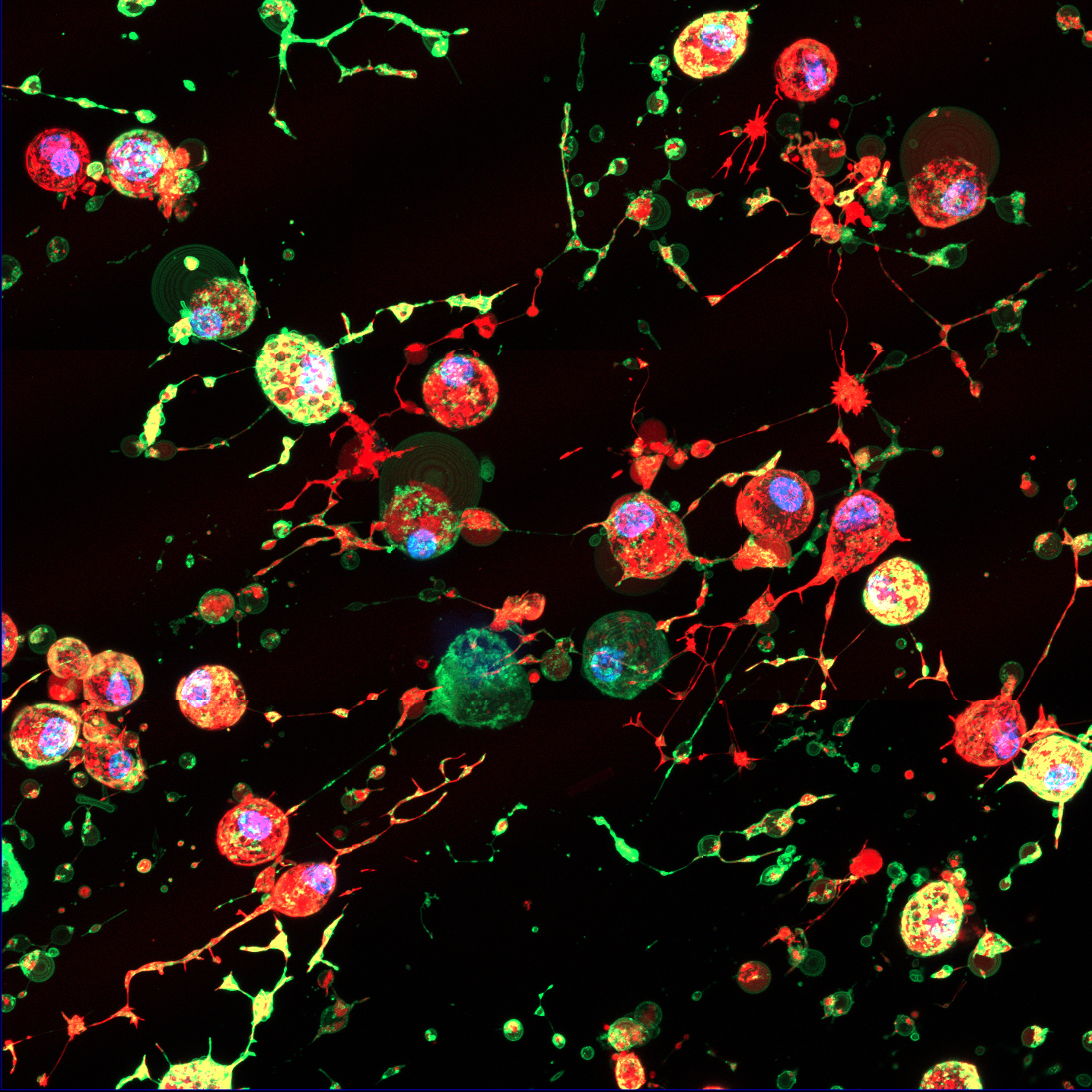
Researchers discovered that as cells self-destruct, they change shape, lift away from their surroundings, and leave behind a residue dubbed “the footprint of death” which contains a previously undiscovered type of Extracellular Vesicle (EV).
EVs are tiny packages released by cells to transport proteins, lipids, DNA and RNA to other cells, serving as a crucial mechanism for communication between cells.
The new EVs, known as F-ApoEVs, mark the site of a dead cell and serve as breadcrumb clues to help the immune system identify and clean up cell fragments, preventing unwanted inflammation.
But early tests on cells also revealed that when dying cells are infected with influenza, the virus can hijack the clean-up process by hiding particles inside the F-ApoEVs, which could aid the spread of infection to neighbouring cells.
Professor Poon, Director of the Research Centre for Extracellular Vesicles (RCEV), said the findings could have a big impact on future drug development.
“Understanding this basic biological process could open new avenues of research to develop new treatments that harness these steps and help the immune system better fight disease,” Professor Poon said.
“Billions of cells are programmed to die each day as a part of normal turnover and disease progression, and until now, it was believed that the cell fragmentation process during cell death was random and fairly simple.
“Our findings demonstrate the complexity of this process and highlight how each step in the process is actually critical to help the dying cell break down efficiently and to be cleared away by the immune system.”
Lead researcher and PhD candidate Stephanie Rutter said the study demonstrated the importance of cell-to-cell communication to maintain health, and how these processes can be manipulated by viruses.
“We know that the body clears away dead cell fragments to prevent them lingering and causing inflammation and autoimmune diseases such as Systemic Lupus Erythematosis (SLE), and we saw F-ApoEVs are readily cleared from the site of cell death,” Stephanie said.
“What we didn’t expect was how viruses can also take advantage of this process and cause infection by hiding in F-ApoEVs.”
Researchers hope the discovery could lead to better understanding and eventually better therapies for infectious and autoimmune disease.
“The more we can understand about cell death and what happens to cells after they die, the better we can understand disease pathologies and find new treatments,” Stephanie said.
The study’s co-leader, Dr Georgia Atkin-Smith from WEHI, said that understanding how dying cells communicated within the immune system was critical, given the broad implication of cell death across many diseases.
“This study has revealed that dying cells can continue to communicate from the grave and may impact immune function,” Dr Atkin-Smith said.
This research was conducted by scientists at La Trobe University’s RCEV, LIMS and the School of Agriculture, Biomedicine and Environment (SABE). The study was conducted in collaboration with researchers at WEHI and Toronto Metropolitan University in Canada.
DOI: 10.1038/s41467-025-64206-3



 Australia; VIC
Australia; VIC