News release
From:
Researchers link Alzheimer’s disease protein to development of preeclampsia
Researchers at Wakayama Medical University in Japan have discovered that amyloid-β deposits disrupt the formation of the placenta and may therefore contribute to the development of preeclampsia during pregnancy. These amyloid-β deposits are similar to those characteristically found in the brains of Alzheimer’s disease patients. The findings were published January 20 in the journal Life Science Alliance.
Preeclampsia is a severe complication that occurs in up to 5% of pregnancies, resulting in high blood pressure and organ damage that can threaten the life of both the mother and fetus. The underlying cause of preeclampsia is unclear, though it has been linked to reduced oxygen levels (hypoxia) and defects in placenta formation. At present, there are no treatments for the condition except for delivery of the fetus, and preeclampsia is the leading cause of medically-induced premature birth.
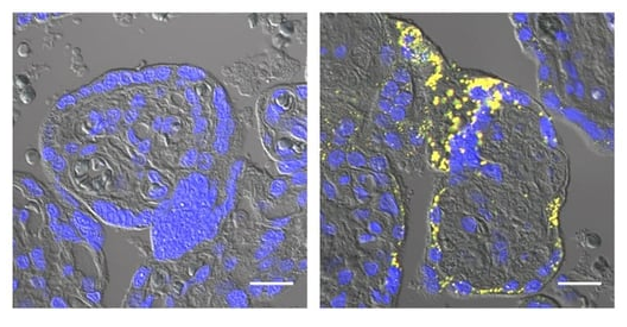
Several previous studies including Buhimschi et al, 2014, Cater et al, 2019, and Cheng et al, 2021, have reported the presence of amyloid-β aggregates in preeclamptic placentas. Why amyloid-β accumulates under these conditions, and whether this impacts placental function and the development of preeclampsia, was unknown.
Hypoxia is known to inhibit a critical step in placenta formation known as syncytialization. During this step, placental stem cells fuse together to form the syncytiotrophoblast, the outer, multinucleated layer of the placenta that protects the developing fetus, produces crucial pregnancy hormones, and mediates the exchange of nutrients, gases, and waste between the mother and the fetus. Nishitsuji and colleagues found that amyloid-β aggregates impair the ability of placental cells grown in the lab to undergo syncytialization. In utero, this would likely inhibit syncytiotrophoblast formation and contribute to the development of preeclampsia.
“Additional studies using animal models will be necessary to elucidate the significance of amyloid-β production and deposition in the physiology and pathology of the placenta,” Nishitsuji says. “Understanding the mechanisms underlying placental defects is important for the prevention of preeclampsia and development of novel preeclampsia therapies.”



 International
International