News release
From:
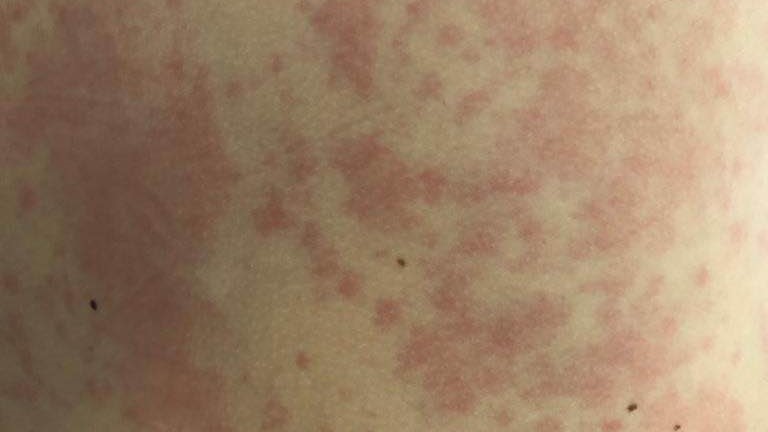
Unexpected urticarial reactions (hives) seen in trial of HIV mRNA vaccines
A safety analysis of mRNA vaccination in a phase 1, randomized, open-label clinical trial evaluated the safety and tolerability of three investigational HIV-1 trimer mRNA vaccines. The study vaccines were found to be generally safe and tolerable. However, the researchers observed unexpected, delayed onset urticaria, or hives, in 7% of participants, highlighting the importance of promoting awareness and reporting of chronic urticaria after mRNA vaccination, adopting risk mitigation strategies in future mRNA vaccine trials, and encouraging further evaluation to determine the cause of these reactions. Urticarial reactions, mostly transient, have been reported with both licensed mRNA COVID-19 vaccines. The study is published in Annals of Internal Medicine.
A team of researchers funded by the National Institutes of Health analyzed the safety and tolerability of three investigational HIV-1 trimer mRNA vaccines: BG505 MD39.3 gp140 (soluble trimer gp140); BG505 MD39.3 gp151 (membrane-bound trimer gp151); and BG505 MD39.3 gp151 CD4 knockout (membrane-bound gp151 CD4KO). 108 participants without HIV aged 18 to 55 years were enrolled at 10 US clinical research sites between February and August 2022 and were randomly assigned to receive one of the three vaccines. Vaccines were administered intramuscularly at weeks 0, 8, and 24. Participants were observed for at least 30 minutes after vaccination and completed a daily symptom diary for a week after. Participants reported frequent but mild to moderate side effects including pain at the injection site, fatigue and muscle aches that were like those seen previously with licensed mRNA COVID-19 vaccines. 80 participants reported 180 adverse events (AEs), with 30 AEs related to the study vaccinations. These AEs included lymphadenopathy, axillary pain, and angioedema. Urticaria was observed in seven participants, four of which had unresolved, intermittent urticaria at 12 months. All seven participants with urticaria had prior receipt of mRNA COVID-19 vaccines, and urticaria occurred with each of the three study vaccines. The study details the first two cases of participants with delayed onset, generalized urticaria with dermographism related to study products. The mRNA platform is under investigation in many vaccines, including HIV-1 vaccines. While the mechanism behind urticarial reactions with these investigational HIV-1 mRNA vaccines is currently unknown, the researchers note that mRNA technology continues to hold great promise for vaccines and therapeutics.



 International
International