News release
From:
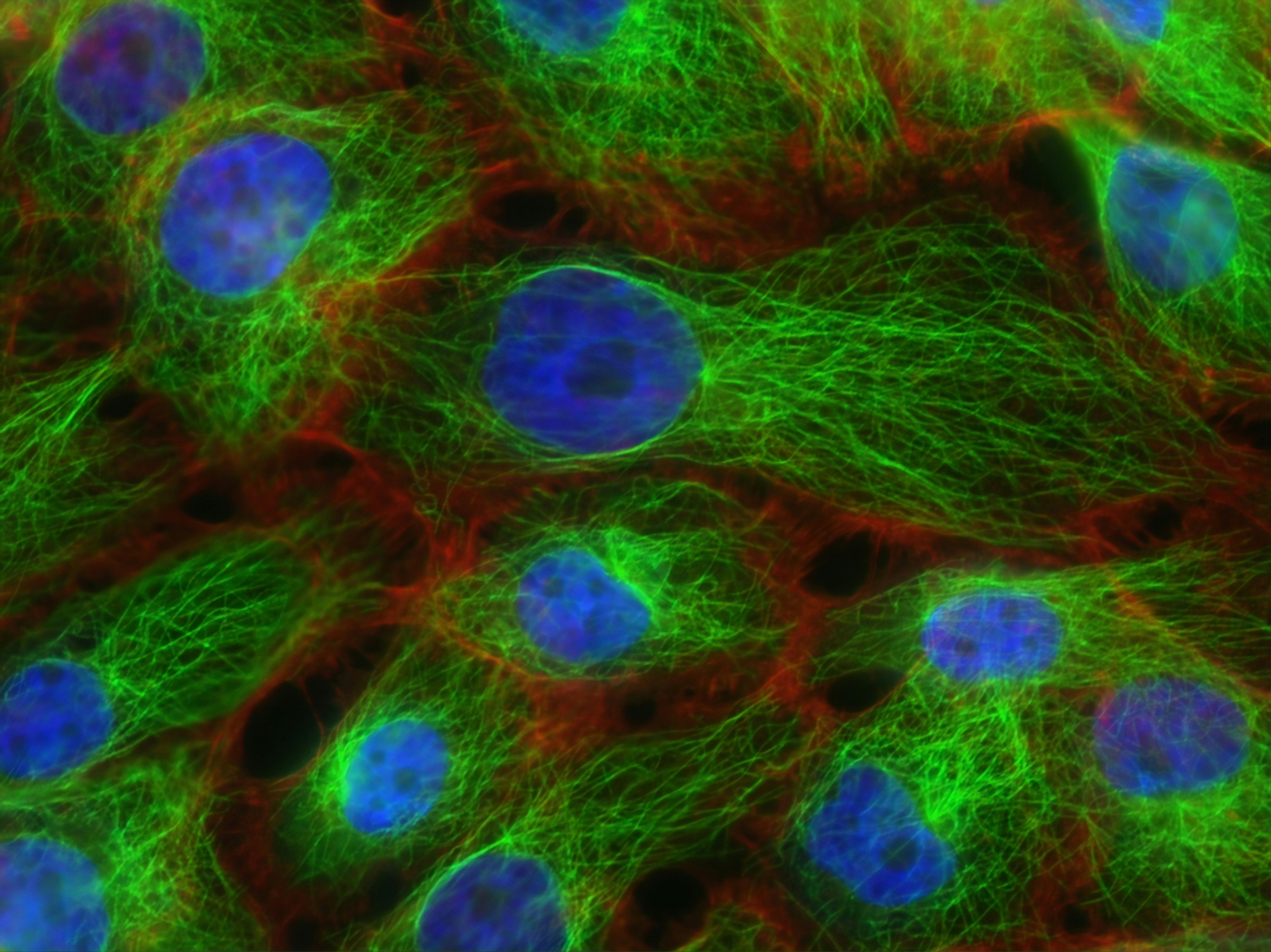
- Cell therapy manufacturing – A new approach for separating cells holds promise for cheaper and more efficient manufacture of cell therapies to treat disease. A prototype 'inertial focusing microfluidic device', which filters cells through a spiral of microchannels, effectively separated target cells quickly without damage. This could reduce the time, cost and complexity of clinical cell therapy manufacturing over conventional methods like centrifuging. Interface
Human leukocytes processed by fast-rate inertial microfluidics retain conventional functional characteristics
Journal of the Royal Society Interface
Cell therapies are medicines made from human cells. Often, scientists use a process called centrifugation in manufacturing of cell therapies, but this can be slow and other alternatives are expensive. We made a new prototype ‘inertial focusing microfluidic device’ that has the potential to be integrated into cell therapy manufacturing procedures. Our device processes cells at a very fast rate and at low cost. It was previously unknown if cells tolerate the large forces experienced in similar devices, but our experiments showed that the cells appeared to be as healthy as centrifuged cells.



 International
International