News release
From:
A genetic variant known as APOE ε4 has, until now, been considered the strongest genetic risk factor for late-onset Alzheimer’s disease.
A new study, published in Nature Medicine, reveals that APOE ε4 functions as a broad immune modulator, creating a built-in vulnerability to a range of neurodegenerative diseases, not just Alzheimer’s.
This discovery lays the foundation for developing precision biomarkers and targeted intervention strategies for a range of neurodegenerative diseases.
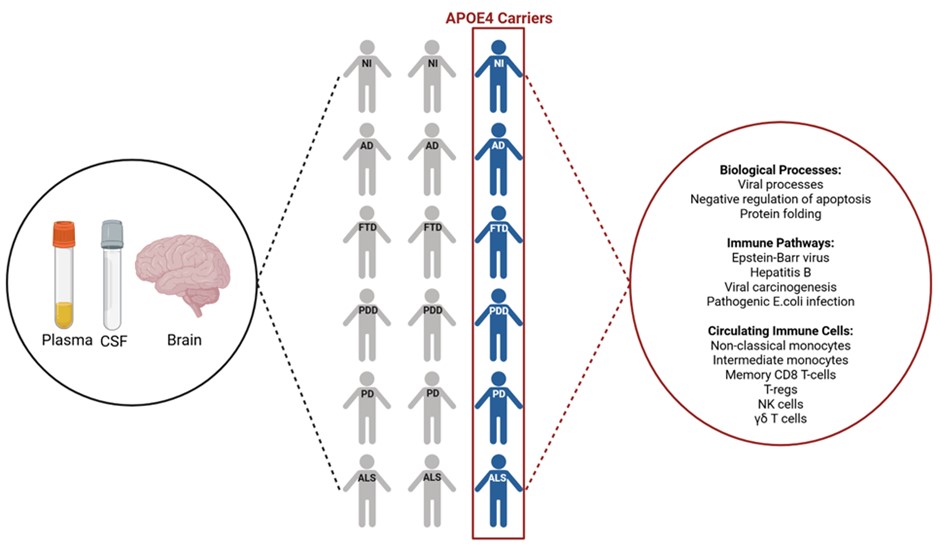
Led by Dr Caitlin Finney, Head of the Neurodegeneration and Disease Modelling Lab at The Westmead Institute for Medical Research (WIMR), the team analysed 1,346 cerebrospinal fluid and 9,924 plasma samples from more than 20 international cohorts using data from the new Global Neurodegeneration Proteomics Consortium.
Dr Finney says, “Our team used machine learning to undertake a large-scale study of proteins. We were able to identify a consistent pattern of proteins (called a signature) that is shared by APOE ε4 carriers with Alzheimer’s disease, frontotemporal dementia, Parkinson’s disease dementia, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), as well as those aging normally.”
While the overall immune-related protein signature was shared by all APOE ε4 carriers, some of the individual proteins within that signature had different links to clinical and lifestyle factors, like blood pressure or smoking, depending on the specific disease.
The team’s Senior Bioinformatician and co-lead of the study, Dr Artur Shvetcov says, “Our findings show that while the presence of the APOE ε4 genetic variant increases vulnerability to neurodegeneration, it is not sufficient on its own to cause disease. The signature we observed points to a chronic pro-inflammatory immune state that spans the plasma, cerebrospinal fluid, and brain”.
Dr Finney adds, “These findings emphasise the importance of understanding how the APOE ε4 genetic variant interacts with a person’s environment, medical history, and lifestyle to influence neurodegenerative disease risk and progression.
By demonstrating that APOE ε4 contributes to a shared immune mechanism across multiple neurodegenerative diseases, this study challenges the long-held notion that its effects are specific to Alzheimer’s disease.
“We believe this work provides a critical foundation for developing blood-based biomarkers and targeted therapies to individuals carrying APOE ε4. Ultimately, it may help enable earlier diagnosis and precision prevention strategies across a range of neurodegenerative conditions.”
Diseases associated with APOE ε4, including Alzheimer’s disease and Parkinson’s disease, affect millions of people and place a significant burden on healthcare systems globally.
The WIMR team is now working to translate these large-scale clinical findings into laboratory-based studies to pinpoint the underlying biological mechanisms, identify early biomarkers, and test new precision medicine therapies.
About the Global Neurodegeneration Proteomics Consortium (GNPC):
The Global Neurodegeneration Proteomics Consortium (GNPC) is a global initiative (and public-private partnership) uniting academic and industry researchers to accelerate biomarker discovery and therapeutic innovation for aging and neurodegenerative diseases, including Alzheimer’s Disease, Parkinson’s Disease, ALS, and FTD.
The Global Neurodegeneration Proteomics Consortium (GNPC) was formed in 2023 when a group of 20+ institutions studying neurodegenerative diseases came together with Gates Ventures and Johnson & Johnson to make it easier to aggregate, harmonise, and share scientific data at the scale needed to generate breakthrough insights. A list of member institutions is available at https://neuroproteome.org/cohorts.
Multimedia




 Australia; International; NSW
Australia; International; NSW