News release
From:
Neuroscience: Identifying neurons that restore walking after paralysis (N&V)
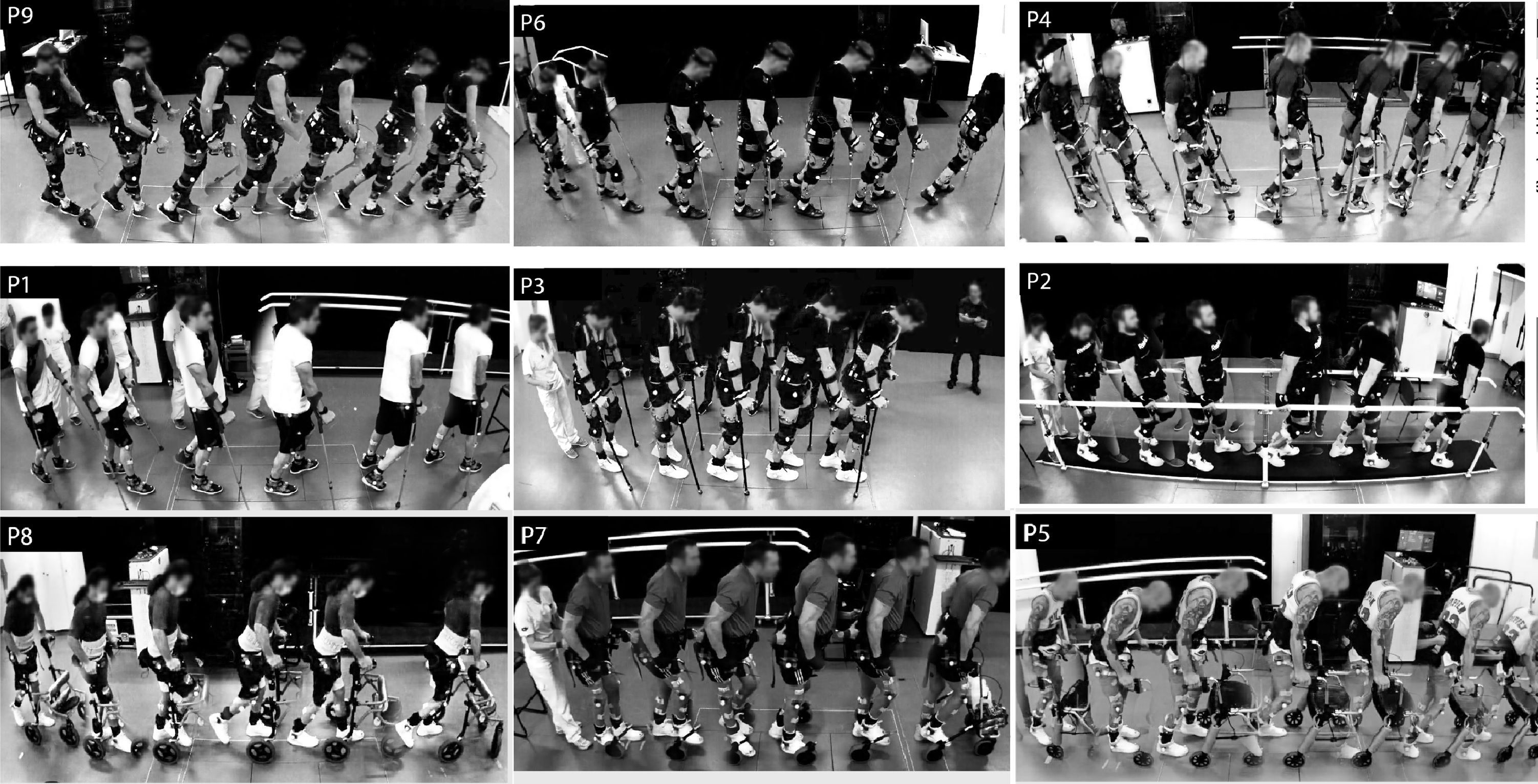
The neurons that promote recovery from paralysis are identified in a study in which nine individuals with chronic spinal cord injury regained the ability to walk after being treated with electrical stimulation. The findings, presented in a Nature paper this week, improve our understanding of how mobility can be recovered after paralysis.
Electrical stimulation of the spinal cord has been found to be effective in improving recovery of walking in people with paralysis, but the underlying mechanism of this treatment remains unclear.
Grégoire Courtine and colleagues investigated whether electrical stimulation might recruit specific sets of neurons in the spinal cord that become necessary for patients to walk after paralysis. In this study, nine individuals with severe or complete paralysis caused by spinal cord injury were enrolled in a clinical trial and received epidural electrical stimulation (EES) treatment. All patients immediately regained or improved their ability to walk during the treatment and showed improvements in mobility after five months of EES treatment and rehabilitation. To explore the underlying mechanism of this improvement, the authors developed a mouse model that replicates the key features of EES neurorehabilitation in humans. In addition, they established a single-cell map of gene expression in various neurons of the mouse spinal cord. Combining the model and the molecular map, the authors identified a specific type of excitatory neuron that plays an important role in restoration of walking after spinal cord injury but is not necessary for walking in individuals without spinal cord injury.
The findings bring us a step closer to understanding the mechanisms of EES rehabilitation. However, the authors note that other neurons in the brain and spinal cord contribute to the recovery of walking, and therefore further studies are needed.



 International
International