News release
From:
Springer Nature
Cancer: Responses and resistance to a new potential drug for acute leukaemia
Anticancer effects and possible signatures of treatment resistance are reported in a phase 1 human clinical trial of a new drug known as revumenib for the treatment of patients with advanced or treatment-resistant acute myeloid leukaemia. The findings are presented in two papers published in Nature this week.
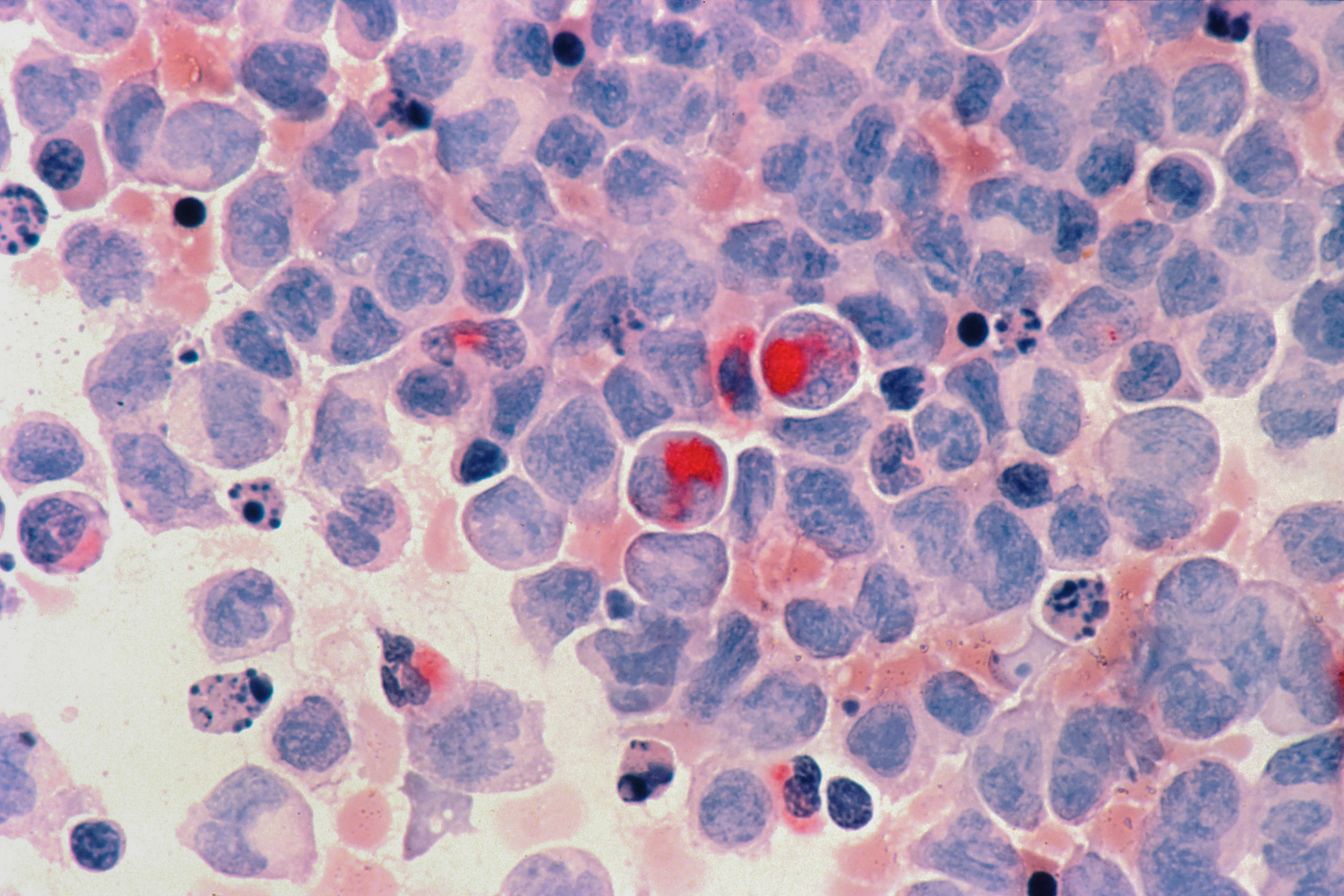
Acute leukaemia is commonly characterized by the mutation of the nucleophosmin 1 (NPM1) gene or rearrangement of the mixed lineage leukaemia 1 gene (KMT2Ar), both of which have been shown to contribute to cancer progression. Overall survival rates are poor and there are currently no approved treatments that specifically target these gene alterations. Preclinical studies have demonstrated that a protein known as menin facilitates the progression of KMT2Ar or NPM1-mutated acute leukaemia, indicating that menin inhibition may reverse cancer progression in this subset of leukaemia.
Ghayas Issa and colleagues report the outcomes of treatment with revumenib, a selective inhibitor of menin, in 60 patients between 2019 and 2022. They report an overall response rate (some degree of remission) of 53%, with a 30% rate (18 out of 60 patients) of complete remission or complete remission with partial haematologic recovery. Of these 18 patients, 78% had undetectable measurable residual disease after nearly two months of remission These results provide evidence of the potential of menin-inhibitor treatments for acute leukaemia.
In a second study, Scott Armstrong and colleagues investigated the occurrence of selective resistance to menin inhibition. They identified specific mutations in the MEN1 gene (which encodes menin) that can result in resistance to revumenib treatment through alteration of the drug-binding site. These mutations were detected in several patients who initially responded to treatment with revumenib but failed to sustain clinical responses. Identification of these treatment-escape routes provides valuable information that will be required to improve patient outcomes in the future.
Attachments
Note: Not all attachments are visible to the general public.
Research URLs will go live after the embargo ends.

Research
Springer Nature, Web page
Paper 1. The URL will go live after the embargo ends

Research
Springer Nature, Web page
Paper 2 - The URL will go live after the embargo ends
Journal/
conference:
Nature
Organisation/s:
Paper 1: The University of Texas, USA. Paper 2: Boston Children’s Hospital and Harvard Medical School, USA
Funder:
Paper 1: This study was funded by Syndax Pharmaceuticals. Paper 2: This research
was funded in part through the NIH/NCI Cancer Center support grant P30 CA008748. S.F.C.
was supported by a Scholar Award from the American Society of Hematology, a Momentum
Fellowship Award from The Mark Foundation for Cancer Research, a Young Investigator Award
from the Edward P. Evans Foundation, and a Career Development Award from the NCI (K08
CA241371-01A1). S.A.A. was supported by NIH grants CA176745, CA206963, CA204639 and
CA066996. S.A.A. and R.M.S. were supported by a SPORE grant in myeloid malignancies
(P50CA206963). R.L.L. was supported by a Cycle For Survival Innovation grant, NCI grants R35
CA197594 and R01 CA173636, a grant from the Samuel Waxman Cancer Research Foundation,
funding support from the Martino Family Foundation, and SCOR grants from the Leukemia
and Lymphoma Society. R.L.L. and S.A.A. were supported by an Alex’s Lemonade Stand
Foundation Crazy 8 grant. F.P. was supported by the German Research Foundation (DFG; PE
3217/1-1), a Momentum Fellowship award by the Mark Foundation for Cancer Research,
a research grant from the ‘Else Kröner-Fresenius-Stiftung’ (2021-EKEA.111), and startup funding
from the University Medicine Greifswald (FOVB-2022-01). C.M. was funded by a Momentum
Fellowship award by the Mark Foundation for Cancer Research. J.D.C. acknowledges support
from NIH grants P30 CA008748 and R01 GM121505. J.D.C., A.V., D.S. and S.S. acknowledge
funding from the Stiftung Charité, the BIH Einstein Foundation, MSKCC, NIH grant R01
GM121505 and Bayer. E.S.F. was supported by NIH grants CA214608 and CA066966. J.A.C. is
supported by a Ruth L. Kirschstein Postdoctoral Individual National Research Service Award
(NIH F32CA250240-02). W.X. was supported by Alex’s Lemonade Stand Foundation and the
Runx1 Research Program, the Cycle for Survival’s Equinox Innovation Award in Rare Cancers,
MSK Leukemia SPORE Career Enhancement Program and a career development award from
the NCI (K08CA267058). H.R. was supported by a Fellow award from the Leukemia and
Lymphoma Society. S.S. is a Damon Runyon Quantitative Biology Fellow supported by the
Damon Runyon Cancer Research Foundation (DRQ-14-22).



 International
International