Media release
From:
A cancer-targeting antibody that helps the body’s immune cells spot and destroy hard-to-treat tumours such as triple-negative breast cancer has been developed by researchers.
The University of Queensland’s Associate Professor Fernando Guimaraes said the antibody recognises a unique part of the ROR1 protein, which is found on many aggressive cancers but rarely on healthy cells.
“The antibody precisely targets cancer cells, helping the immune system kill cancer more effectively while aiming to spare healthy tissue,’’ Dr Guimaraes said.
“This could translate to treatments that are both more effective and gentler.’’
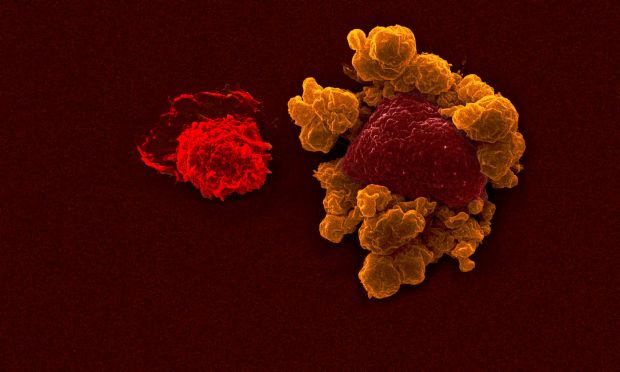
Dr Guimaraes, whose group at the Frazer Institute led the research, said the new antibody activated natural killer (NK) cells – a type of immune cell that destroys tumours.
The researchers found the antibody worked best when combined with treatment that blocked a cancer immuno-suppressing signal – Transforming Growth Factor-beta or TGF-β.
“We engineered ‘super NK cells’ to boost cancer control,’’ Dr Guimaraes said.
“By giving NK cells a genetic upgrade and making them resistant to TGF-β, we created enhanced immune cells that were able to find and destroy ROR1-positive tumours more efficiently in both laboratory and animal models.
“Triple negative breast cancer is an aggressive and difficult-to-treat cancer, with limited effective therapeutic options currently available.
“Our results open the door to new immunotherapy options, including an upgraded version of NK cells that are better at finding and killing cancer.’’
Dr Guimaraes said the results provide a foundation for future research into clinical applications.
“If successful in people, this approach could improve survival and quality of life by shrinking tumours with fewer side effects than some current therapies,’’ he said.
“In practical terms this could lead to clinical trials and, longer term, new treatment choices for patients who currently have few.’’
The research is published in Molecular Therapy.



 Australia; QLD
Australia; QLD