Media Release
From: The University of New South WalesStem cell therapies capable of regenerating any human tissue damaged by injury, disease or ageing could be available within a few years, following landmark research led by UNSW Australia researchers.
The repair system, similar to the method used by salamanders to regenerate limbs, could be used to repair everything from spinal discs to bone fractures, and has the potential to transform current treatment approaches to regenerative medicine.
The UNSW-led research has been published today in the Proceedings of the National Academy of Sciences journal.
Study lead author, haematologist and UNSW Associate Professor John Pimanda, said the new technique, which reprograms bone and fat cells into induced multipotent stem cells (iMS), has been successfully demonstrated in mice.
“This technique is a significant advance on many of the current unproven stem cell therapies, which have shown little or no objective evidence they contribute directly to new tissue formation,” Associate Professor Pimanda said.
“We are currently assessing whether adult human fat cells reprogrammed into iMS cells can safely repair damaged tissue in mice, with human trials expected to begin in late 2017.”
There are different types of stem cells including embryonic stem (ES) cells, which during embryonic development generate every type of cell in the human body, and adult stem cells, which are tissue-specific. There are no adult stem cells that regenerate multiple tissue types.
“This technique is ground-breaking because iMS cells regenerate multiple tissue types,” Associate Professor Pimanda said.
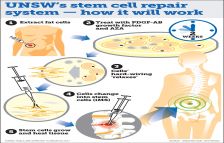
“We have taken bone and fat cells, switched off their memory and converted them into stem cells so they can repair different cell types once they are put back inside the body.”
The technique developed by UNSW researchers involves extracting adult human fat cells and treating them with the compound 5-Azacytidine (AZA), along with platelet-derived growth factor-AB (PDGF-AB) for approximately two days. The cells are then treated with the growth factor alone for a further two-three weeks.
AZA is known to induce cell plasticity, which is crucial for reprogramming cells. The AZA compound relaxes the hard-wiring of the cell, which is expanded by the growth factor, transforming the bone and fat cells into iMS cells. When the stem cells are inserted into the damaged tissue site, they multiply, promoting growth and healing.
The new technique is similar to salamander limb regeneration, which is also dependent on the plasticity of differentiated cells, which can repair multiple tissue types, depending on which body part needs replacing.
The study’s first author, Dr Vashe Chandrakanthan, who developed the technology, said the new technique is an advance on other stem cell therapies being investigated, which have a number of deficiencies.
“Embryonic stem cells cannot be used to treat damaged tissues because of their tumour forming capacity. The other problem when generating stem cells is the requirement to use viruses to transform cells into stem cells, which is clinically unacceptable,” Dr Chandrakanthan said.
“We believe we’ve overcome these issues with this new technique.”
Neurosurgeon and Conjoint Lecturer with UNSW’s Prince of Wales Clinical School, Dr Ralph Mobbs, will lead the human trials, once the safety and effectiveness of the technique using human cells in mice has been demonstrated.
“The therapy has enormous potential for treating back and neck pain, spinal disc injury, joint and muscle degeneration and could also speed up recovery following complex surgeries where bones and joints need to integrate with the body,” Dr Mobbs said.
Research shows that up to 20% of spinal implants either don’t heal or there is delayed healing. The rates are higher for smokers, older people and patients with diseases such diabetes or kidney disease.
“Spinal implants currently used to replace damaged or troubled discs don’t always weld with the adjacent bones, so by transplanting these reprogrammed stem cells, we hope to be able to better fuse these implants to the host bone,” Dr Mobbs said.
“This represents a potential huge leap forward for spinal and orthopaedic procedures.”
Along with confirming that human adult fat cells reprogrammed into iMS stem cells can safely repair damaged tissue in mice, the researchers said further work is required to establish whether iMS cells remain dormant at the sites of transplantation and retain their capacity to proliferate on demand.
Expert Reaction
These comments have been collated by the Science Media Centre to provide a variety of expert perspectives on this issue. Feel free to use these quotes in your stories. Views expressed are the personal opinions of the experts named. They do not represent the views of the SMC or any other organisation unless specifically stated.